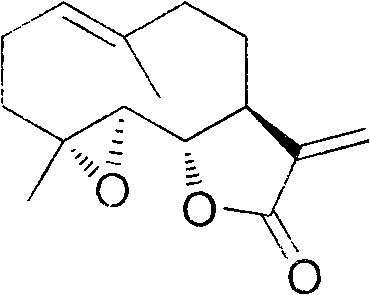
Method for preparing parthenolide originated from magnolia delavayi, magnolia liliiflora and magnolia denudate
A technology of parthenolide and magnolia, which is applied in the direction of organic chemistry, can solve the problems of cumbersome process, high production cost, and not too high parthenolide content, and achieve the effect of easy industrialization and simple extraction and separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Isolation and purification of parthenolide from the root bark of Yulan magnolia
[0027] Take 1 kg of dry root bark of Magnolia japonica, and grind it into powder. Get the pulverized coarse powder, extract 3 times with 10 times (weight) of 95% ethanol in reflux, each time for 3 hours. Filtrate, combine the filtrates, and recover the solvent under reduced pressure to obtain the extract. The extract was dissolved with 20% ethanol, then extracted with ethyl acetate until the color of the extract became lighter, the organic solvent was combined, the solvent was recovered under reduced pressure, and dried to obtain 114 grams of solid. Put on a silica gel chromatography column, elute with petroleum ether: ethyl acetate = 15:1 ~ 4:1 gradient, TLC tracking detection, compare with standard parthenolide, collect fractions containing parthenolide, combine and reduce pressure The solvent was recovered to obtain a dry solid. Crystallization in a mixed solvent of petrol...
Embodiment 2
[0031] Example 2: Isolation and Purification of Parthenolide from Magnolia Root Bark
[0032] Take 1 kg of dry root bark of Magnolia japonica, and crush it into powder. Get the pulverized coarse powder, extract 3 times with 10 times (weight) of 95% ethanol in reflux, each time for 3 hours. Filtrate, combine the filtrates, and recover the solvent under reduced pressure to obtain the extract. The extract was dissolved with 20% ethanol, then extracted with ethyl acetate until the color of the extract became lighter, the organic solvent was combined, the solvent was recovered under reduced pressure, and dried to obtain 102 grams of solid. Put on a silica gel chromatography column, elute with petroleum ether: ethyl acetate = 15:1 ~ 4:1 gradient, TLC tracking detection, compare with standard parthenolide, collect fractions containing parthenolide, combine and reduce pressure The solvent was recovered to obtain a dry solid. Crystallization in a mixed solvent of petroleum ether: ac...
Embodiment 3
[0033] Example 3: Isolation and purification of parthenolide from magnolia root bark
[0034] Take 1 kg of dry root bark of Magnolia, and grind it into powder. Get the pulverized coarse powder, extract 3 times with 10 times (weight) of 95% ethanol in reflux, each time for 3 hours. Filtrate, combine the filtrates, and recover the solvent under reduced pressure to obtain the extract. The extract was dissolved with 20% ethanol, then extracted with ethyl acetate until the color of the extract became lighter, the organic solvent was combined, the solvent was recovered under reduced pressure, and dried to obtain 112 grams of solid. Put on a silica gel chromatography column, elute with petroleum ether: ethyl acetate = 15:1 ~ 4:1 gradient, TLC tracking detection, compare with standard parthenolide, collect fractions containing parthenolide, combine and reduce pressure The solvent was recovered to obtain a dry solid. Crystallization in a mixed solvent of petroleum ether: acetone = 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com