Polyurethane block copolymer, preparation method thereof and preparation method of polyurethane block copolymer nano hydrogel
A technology of block copolymer and nano hydrogel, which is applied in the field of polyurethane and achieves the effect of simple and easy method, easy control and good pH sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Correspondingly, the present invention provides a method for preparing a polyurethane block copolymer, including the following steps:
[0063] Under the action of a catalyst, the diol containing 1,2-disulfide heterocycle, the diol containing tertiary amine, and the diisocyanate are pre-reacted in an organic solvent, and then reacted with polyethylene glycol monomethyl ether to obtain polyurethane intercalation. A segment copolymer, the polyurethane block copolymer includes an A block represented by formula (I) and a B block represented by formula (II).
[0064] The present invention uses polyethylene glycol monomethyl ether, diols containing 1,2-disulfide heterocycles, tertiary amine-containing diols and diisocyanates as raw materials. The number average molecular weight of the polyethylene glycol monomethyl ether is preferably 500~10000, more preferably 1500~8000, most preferably 2000~5000;
[0065] The diol containing 1,2-disulfide heterocycle is trans-4,5-dihydroxy-1,2-dit...
Embodiment 1~12
[0086] Add a certain amount of PEG with a molecular weight of 2000 to a dry reaction flask, add anhydrous toluene, and azeotropically remove water at 130°C for 6 hours, drain the remaining toluene under reduced pressure, and cool to room temperature under nitrogen protection ;
[0087] Then add DHDT, HEP, catalyst dibutyl tin dilaurate and appropriate amount of 1,2-dichloroethane into the reaction flask containing PEG, and stir at 75°C until the solid is dissolved;
[0088] Then add HDI into the reaction flask, stir and react for 5 hours at 75°C under nitrogen protection;
[0089] The reaction product was settled with ether, and then the obtained solid was dissolved in CHCl 3 After sedimentation with ether, suction filtration, and drying, a polyurethane block copolymer is obtained.
[0090] The specific raw material monomer formula is shown in Table 1, and the molecular weight characteristics of the polyurethane obtained in Examples 1 to 4 are shown in Table 2.
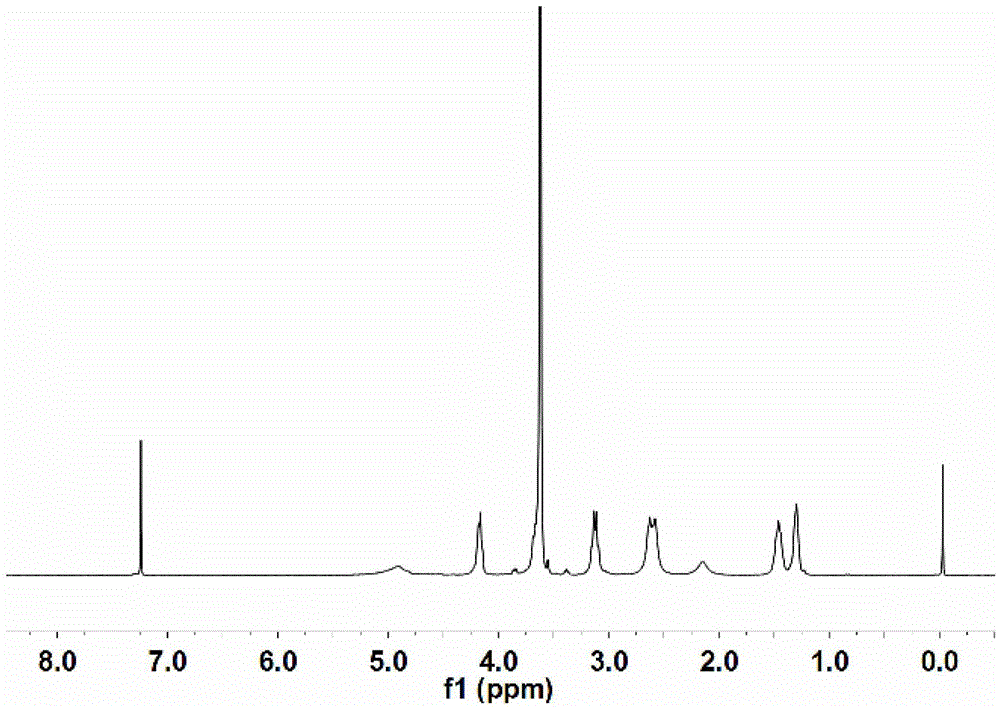
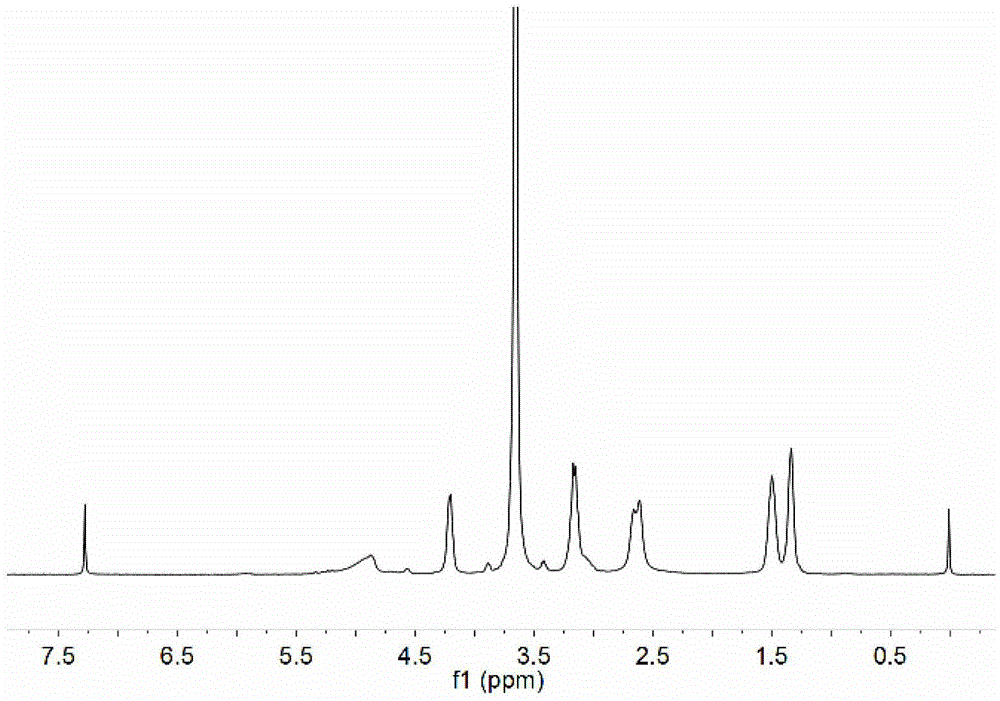
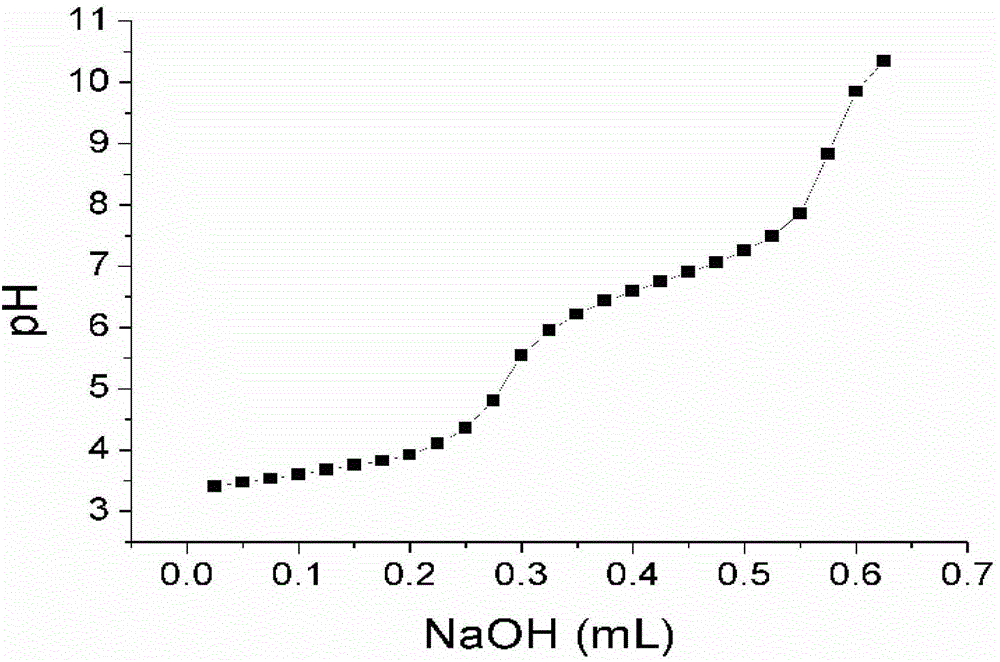
[0091] Perform nuclear...
Embodiment 13
[0094] Add a certain amount of PEG with a molecular weight of 500 to a dry reaction flask, add anhydrous toluene, and azeotropically remove water at 130°C for 8 hours, drain the remaining toluene under reduced pressure, and cool to room temperature under nitrogen protection ;
[0095] Then add DHDT, HEP, catalyst stannous octoate, and appropriate amount of 1,2-dichloroethane into the reaction flask containing PEG, and stir at 75°C until the solid is dissolved;
[0096] Then add TCDI to the reaction flask, stir and react for 5 hours at 75°C under nitrogen protection;
[0097] The reaction product was settled with ether, and then the obtained solid was dissolved in chloroform, and then settled with ether, filtered with suction, and dried to obtain a polyurethane block copolymer. The specific raw material monomer formula is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com