PH-sensitive polyurethane hydrogel as well as preparation method and application thereof
A polyurethane and polyurethane prepolymer technology, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, antibacterial drugs, etc., can solve the problems of wide mutation range, difficult control of mutation range, and insufficient pH sensitivity of the medium. Achieve the effect of fast response, easy promotion, and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
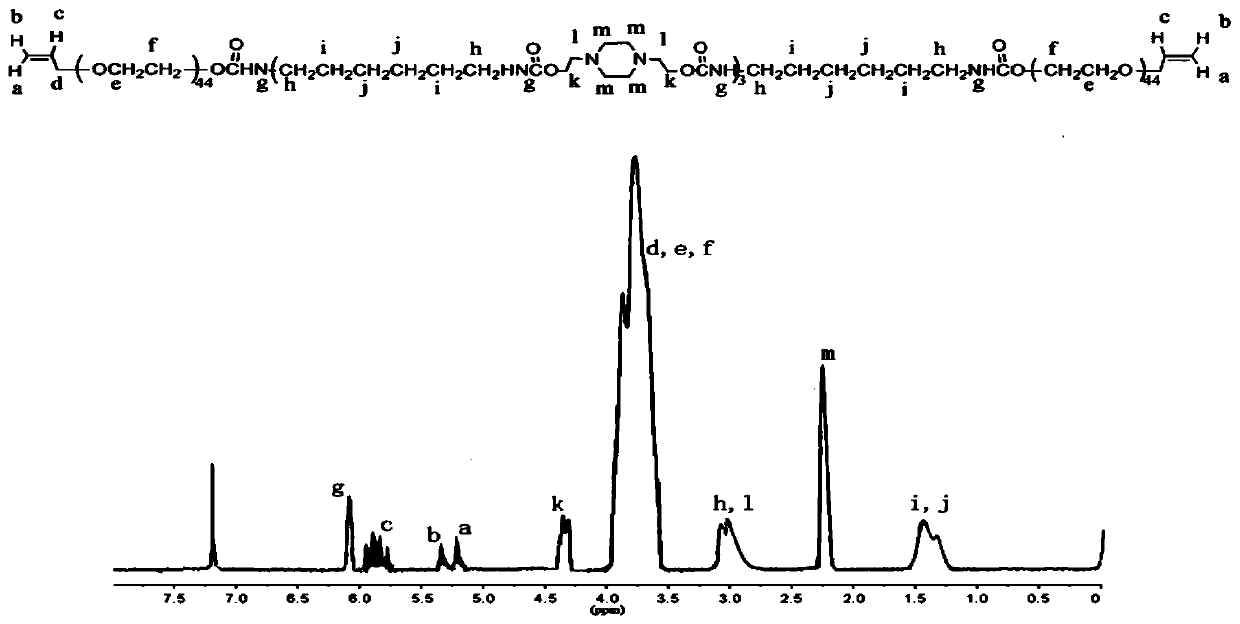
[0046] Dissolve 5.23g N,N'-dihydroxyethylpiperazine, 4.16mL tetramethylene diisocyanate and 0.05g dibutyltin dilaurate in 20mL N,N-dimethylformamide (DMF), heat in an oil bath React at a constant temperature of 75°C until the content of -NCO in the system measured by di-n-butylamine titration reaches the theoretical value, about 2.5 hours. 20.0g of monoallyl polyethylene glycol (number average molecular weight is 2000g / mol) was dissolved in 20mL of DMF solution and added dropwise to the reaction flask, and the reaction was continued at 75°C until the -NCO absorption peak in the FT-IR spectrum disappeared. About 3h. After cooling to room temperature, add a certain amount of DMF solution to dilute the double-ended allyl polyurethane to 0.05g / mL, then settle with eight times the volume of -5°C glacial ether, filter with suction, and dry under vacuum at room temperature to obtain double-ended allyl polyurethane ,That 1 HNMR spectrum see figure 1 .
[0047] Preparation of the g...
Embodiment 2
[0049] Dissolve 5.23g of N,N'-dihydroxyethylpiperazine, 6.41mL of hexamethylene diisocyanate and 0.065g of stannous octoate in 20mL of N,N-dimethylformamide (DMF), and heat the oil bath to 75 ℃ constant temperature reaction until the -NCO content in the system measured by the di-n-butylamine method reaches the theoretical value, about 3 hours. A double-terminated isocyanato polyurethane prepolymer was obtained. 20.0g of monoallyl polyethylene glycol (number average molecular weight is 2000g / mol) was dissolved in 20mL of DMF solution and added dropwise to the reaction flask, and the reaction was continued at 75°C until the -NCO absorption peak in the FT-IR spectrum disappeared. about 3.5h. After cooling to room temperature, add DMF solution to dilute the double-ended allyl polyurethane to 0.05 g / mL, then settle with eight times the volume of -5°C glacial ether, filter with suction, and dry in vacuum at room temperature to obtain double-ended allyl polyurethane.
[0050] Prepa...
Embodiment 3
[0053] Dissolve 5.23g N,N'-dihydroxyethylpiperazine, 8.02mL lysine diisocyanate and 0.08g stannous octoate in 20mL N,N-dimethylformamide (DMF), heat the oil bath to 80 ℃ constant temperature reaction until the -NCO content in the system measured by the di-n-butylamine method reaches the theoretical value, about 3 hours. A double-terminated isocyanato polyurethane prepolymer was obtained. Dissolve 20.0 g of monoallyl polyethylene glycol (number average molecular weight: 2000) in 20 mL of DMF solution and add it dropwise to the reaction flask, and continue the reaction at 80°C until the -NCO absorption peak in the FT-IR spectrum disappears, about 3 hours . After cooling to room temperature, add a certain amount of DMF solution to dilute the double-ended allyl polyurethane to 0.05g / mL, then settle with eight times the volume of -5°C glacial ether, filter with suction, and dry in vacuum to obtain the double-ended allyl polyurethane.
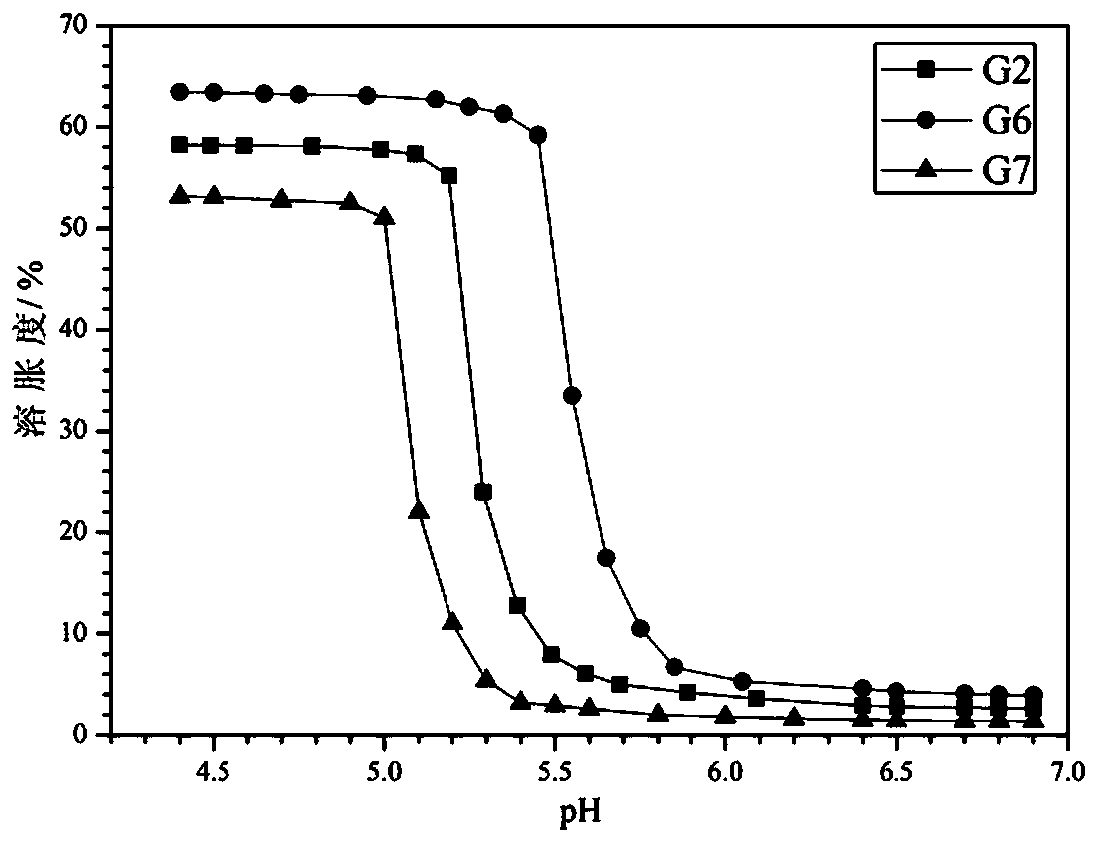
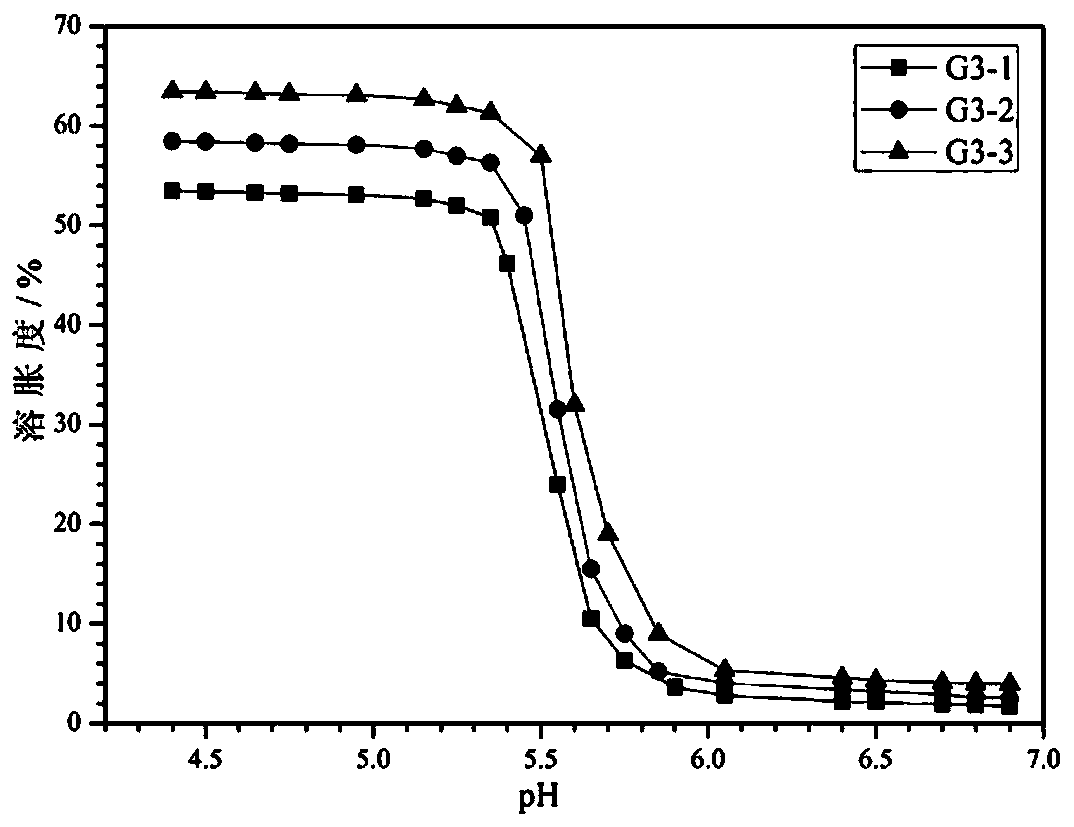
[0054] Gel preparation: 10 g of double-ended...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com