A kind of preparation method of phthalimide-based azo dye
A technology of phthalimide-based azo and dyes, which is applied in the preparation of phthalimide-based azo dyes, two asymmetric structure nitro compounds in the field of preparing azo dyes, can solve a large number of problems Inorganic salt, waste water, environmental impact and other issues, to achieve the effect of high safety, low energy consumption, simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
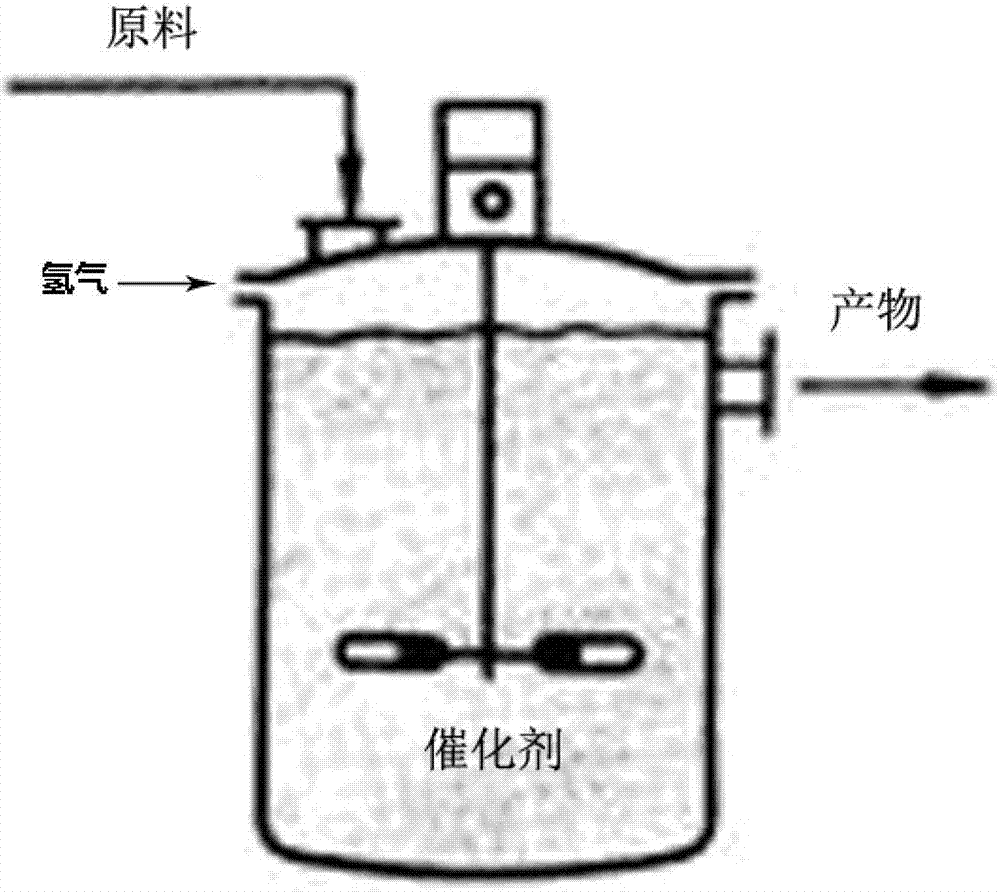
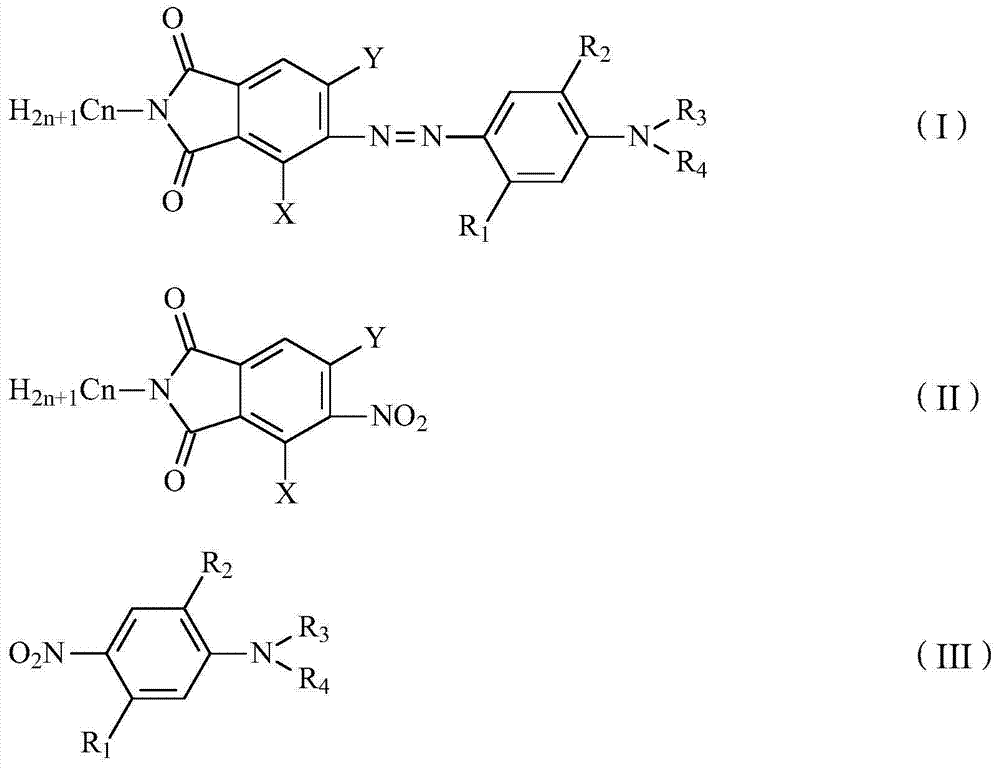
[0017] A preparation method of a phthalimide-based azo dye, comprising mixing a compound of formula (II) and a compound of formula (III) in equimolar proportions, adding them to an alkali-containing solvent, and feeding hydrogen gas in the presence of a catalyst , react at room temperature to 98° C. under low pressure (0.1 to 0.2 MPa) or normal pressure for 4 to 24 hours to directly prepare the compound of formula (I) with an azo structure.
[0018]
[0019]
[0020] Wherein, in formula (I), formula (II) and formula (III), n=1, 2 or 3; X is -H, -CH 3 、-C 2 h 5 , -Cl, -Br; Y is -H, -Cl, -Br; R 1 -H, -CH 3 , -NHCOCH 3 , -NHCOC 2 h 5 ; 2 -H, -CH 3 ; 3 with R 4 Same as -C 2 h 4 OCH 3 、-C 2 h 4 OC 2 h 5 、-C 2 h 4 OCOCH 3 、-C 2 h 4 OCOC 2 h 5 ;
[0021] The catalyst is one of Pt nanomaterials, Pd nanomaterials or a mixture of both.
[0022] The diameter of the catalyst is 2nm-3nm.
[0023] Preferably, the catalyst is used in an amount of 0.01wt%-1wt%...
Embodiment 1
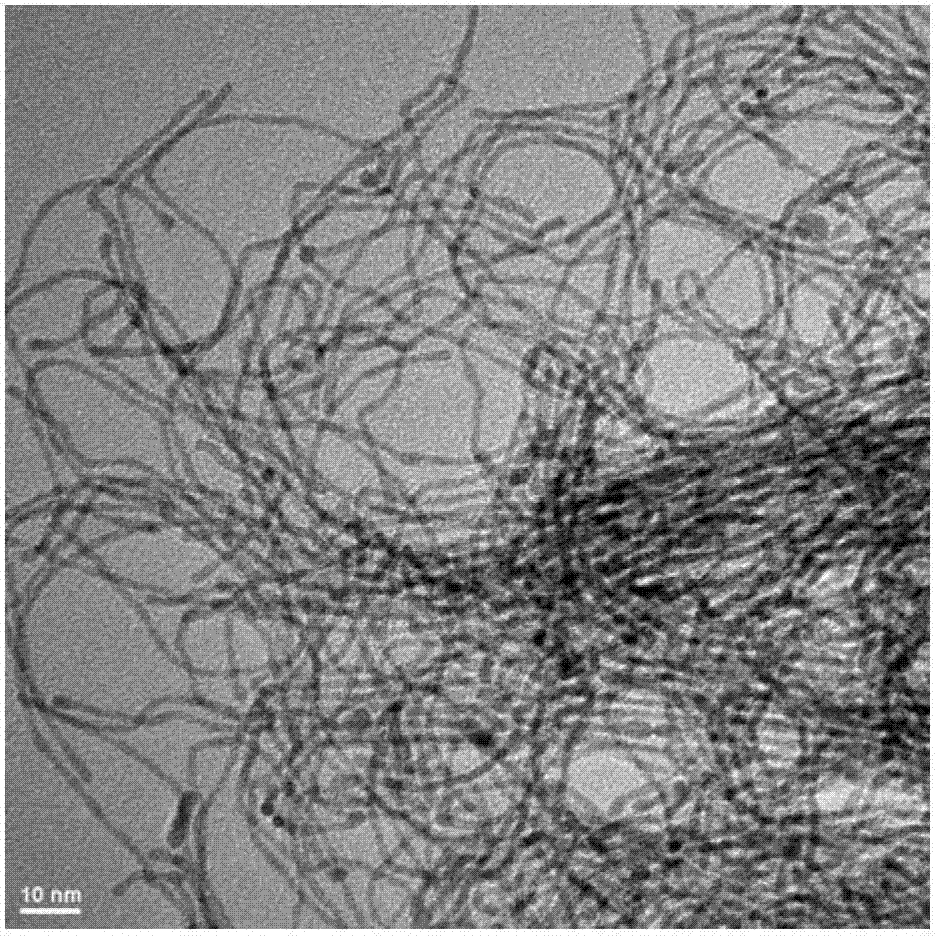
[0029] Synthesis of platinum nanowires: Platinum acetylacetonate was reduced in oleylamine at 160°C while iron pentacarbonyl was thermally decomposed to obtain Fe / Pt nanowires with a diameter of 2nm to 3nm (see Angew.Chem.Int.Ed.46(2007), 6333 -6335), and then heated and stirred under acidic conditions to corrode the Fe wrapped on the outside of the Pt nanowires to obtain platinum nanowires. figure 2 , from attached figure 2 It can be seen that the diameter of the platinum nanowires is 2 nm to 3 nm.
Embodiment 2
[0031] In the reaction bottle of 20g m-xylene, first add 2.85g compound (IIA) and 3.09g compound (IIIA), then add 0.4g sodium hydroxide and 1.4mg platinum nano compound respectively. Put the system into an autoclave, cool, vacuumize, release hydrogen, cycle 3 to 4 times, put in hydrogen, and return to room temperature. The system was kept in an oil bath at 80° C. with a hydrogen pressure of 0.2 Mpa, and the product (IA) was obtained after heating for 15 h with a yield of 78%.
[0032]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com