Method for heterologously expressing active membrane proteins by using Pichia pastoris expression system
A protein and myosin technology, applied in the fields of genetic engineering and enzymes, can solve problems such as difficulties in the expression of membrane protein activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
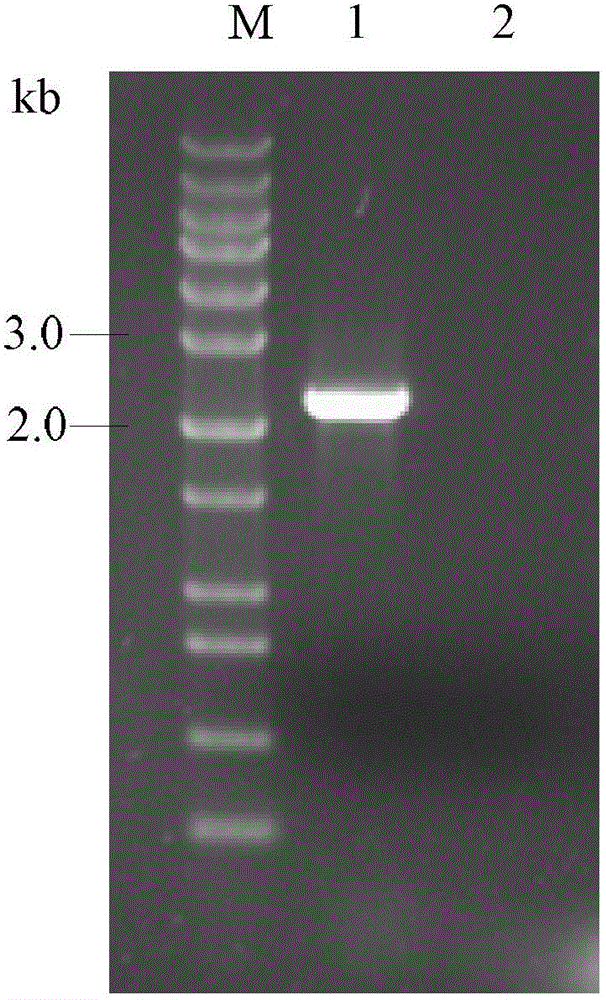
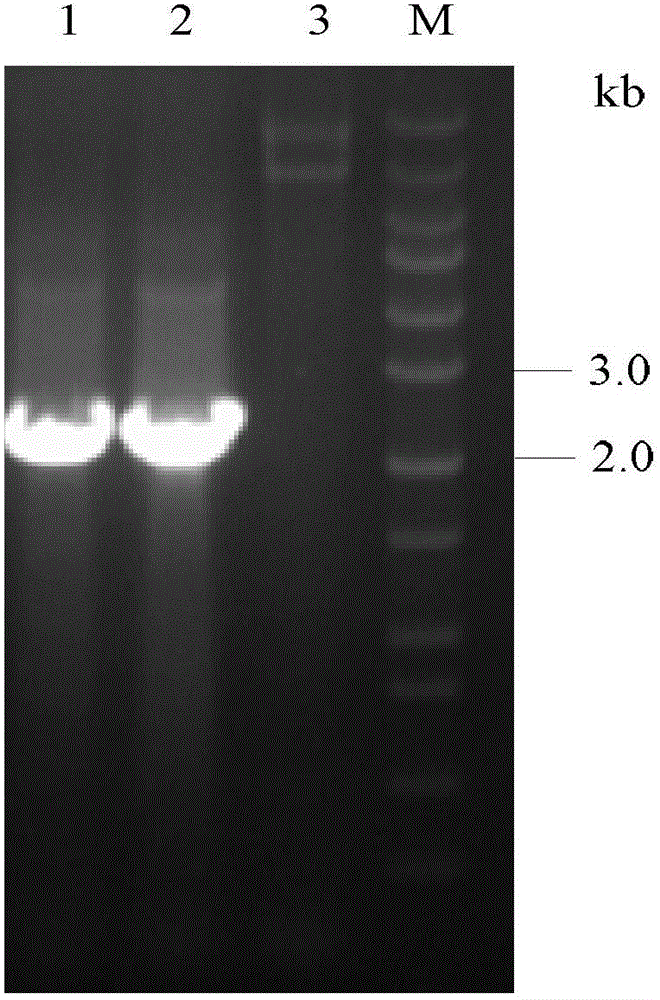
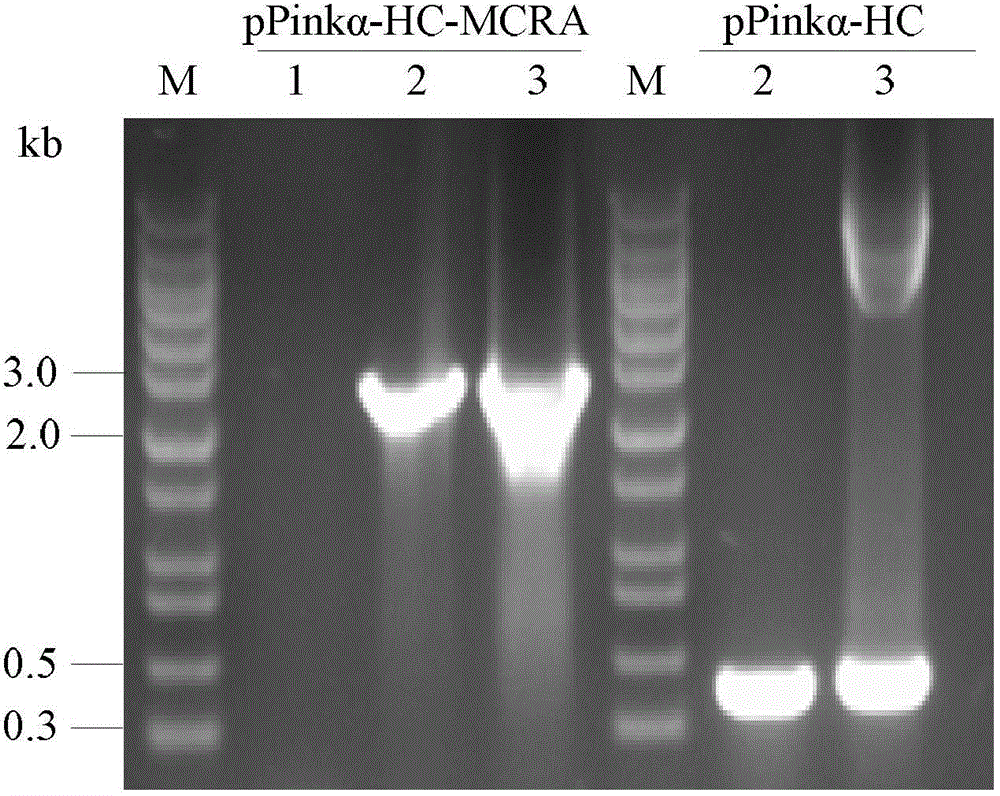
[0029] PichiaPink transfected with the MCRA gene of Bifidobacterium animalis BB-12 TM Establishment of recombinant bacteria.
[0030](1) Test method: Using the genome of Bifidobacterium animalis BB-12 as a template, design primers according to the MCRA sequence of Bifidobacterium animalis BB-12 (GenBank: ADC85468), and use high-fidelity KOD plus enzyme for PCR amplification of MCRA Gene. Using 5'-CCGGATATCATGGACACTAGGGCGCCGAAAGTCG-3', 5'-CGGGGTACCTCAGTGATGGTGATGGTGATGTTTCGCCGAATCATTCTCCCCCG-3' as primers, the MCRA gene was amplified by high-fidelity PCR. The PCR program is: 95°C for 30s, 55°C for 30s, 68°C for 2.5min, 30 cycles. PCR reaction system: 5 μL dNTPs (2mM), 5 μL 10×KOD plus Buffer, 1 μL KOD plus, 2.5 μL MgSO 4 (25mM), 1 μL of upstream and downstream primers, 2 μL of template. The amplified PCR product was digested with EcoR V and Kpn I at 37°C for 3 h, and then ligated with the pPinkα-HC plasmid digested with Stu I and Kpn I at an appropriate ratio. The ligation...
Embodiment 2
[0034] 1 MCRA gene of Bifidobacterium animalis BB-12 in PichiaPink TM Confirmation of expression in recombinant bacteria.
[0035] 1.1 Experimental method: After the recombinant bacteria and control bacteria were induced by methanol, SDS-PAGE and Wester Blot tests were carried out on the proteins of the bacteria and the culture medium.
[0036] (1) Solution preparation:
[0037] BMGY medium: 1% yeast extract, 2% peptone, 100mM potassium phosphate buffer pH6.0, 1.34% YNB, 0.0004% biotin, 1% glycerol; replace glycerol in BMGY with 1% methanol to obtain BMMY culture base;
[0038] (2) The recombinant bacteria and the control bacteria were cultured in BMGY medium at 28°C and 250rpm for 2 days, then centrifuged at 1500g for 5min, removed the BMGY medium, resuspended the bacteria with BMMY medium, and induced culture at 28°C and 250rpm for 2 days. Two days later, the fermentation broth and cells were taken for SDS-PAGE and Western Blot analysis, and the concentration of the separ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com