Recombinant yeast for fermentation production of chondroitin sulfate with controllable molecular weight and application of recombinant yeast
A chondroitin sulfate and yeast technology, applied in the field of bioengineering, can solve problems such as the inability to regulate the molecular weight of chondroitin sulfate, and achieve the effects of avoiding extraction and purification and post-catalytic processes, controllable molecular weight, and quality and safety assurance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
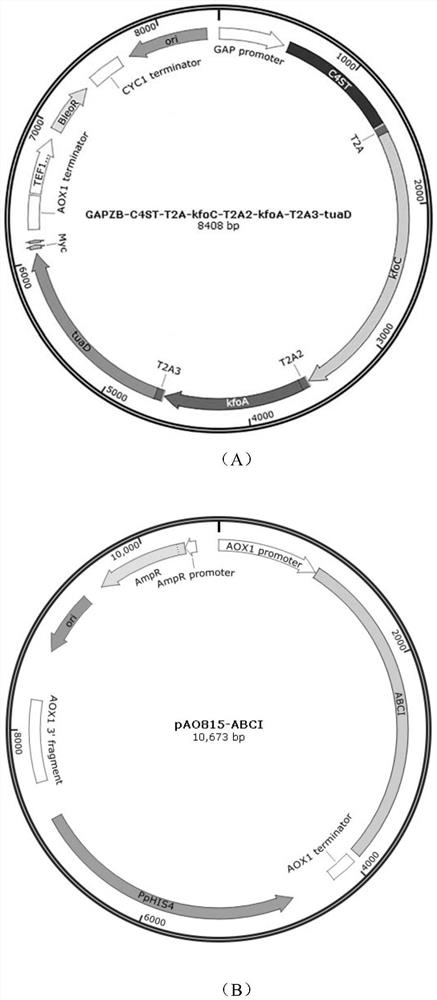
[0043] Example 1: Construction of pGAPZB-C4ST-T2A-kfoC-T2A2-kfoA-T2A3-tuaD plasmid and recombinant strain Pp001
[0044] (1) Using the synthetic genes C4ST, kfoC, kfoA and tuaD (sequences shown in SEQ ID NO: 8, SEQ ID NO.5-7 respectively) as a template, with primers C4-F / C4-R, kfoC-F / Four genes of kfoC-R, kfoA-F / kfoA-R, tuaD-F / tuaD-R were amplified by PCR respectively, and connected to the pGAPZB vector by Gibson assembly to construct pGAPZB-C4ST-T2A-kfoC-T2A2-kfoA -T2A3-tuaD plasmid, wherein T2A, T2A2 and T2A3 amino acid sequences are all SEQ ID NO.2, but have different nucleotide sequences, the nucleotide sequence used by T2A is GAA GGT CGT GGT TCT CTT CTG ACT TGTGGT GAT GTT GAA GAAAAC CCA GGT CCA; the nucleotide sequence used for T2A2 is GAA GGT CGT GGA TCCCTA CTT ACT TGC GGT GAC GTA GAG GAA AAC CCT GGT CCG; the nucleotide sequence used for T2A3 is GAGGGT AGA GGT TCT TTG CTT ACT TGC GGT GAC GTT GAG GAA AAC CCA GGT CCA.
[0045] (2) To prepare competent cells of Pichia pa...
Embodiment 2
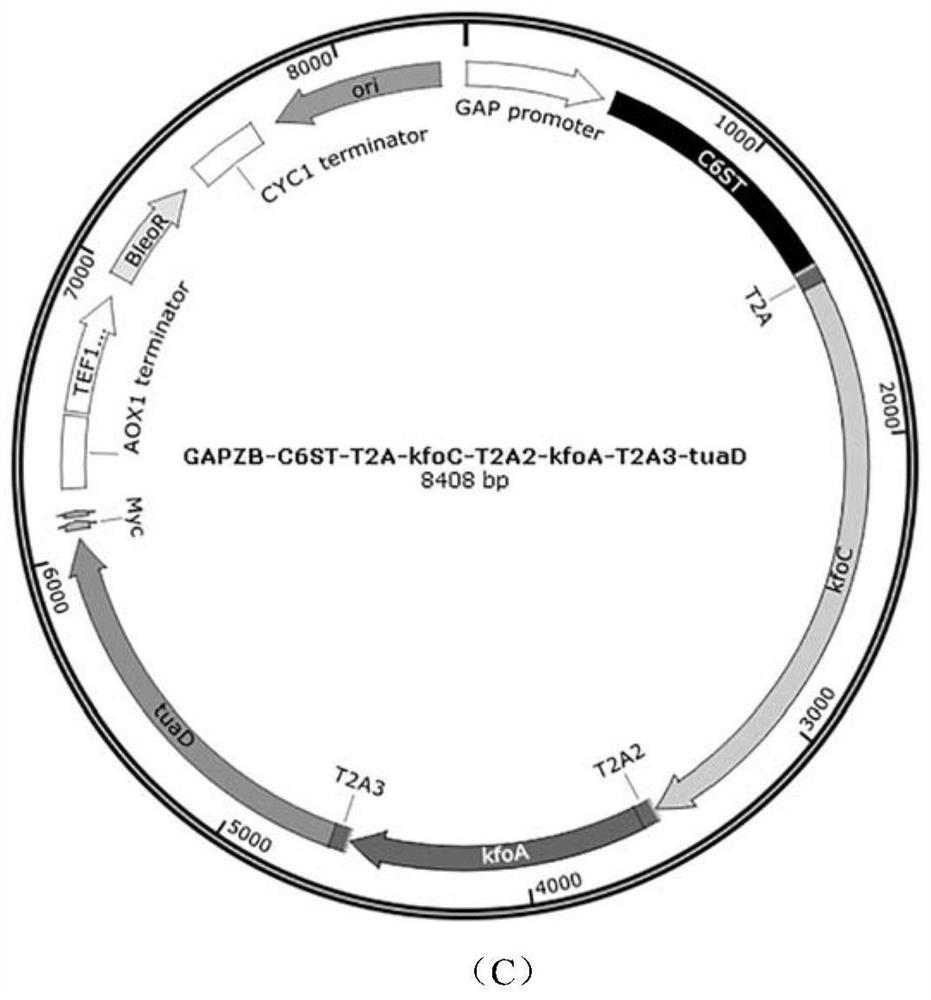
[0046] Example 2: Construction of pGAPZB-C6ST-T2A-kfoC-T2A2-kfoA-T2A3-tuaD plasmid and recombinant strain Pp002
[0047] (1) Using the synthetic genes C6ST, kfoC, kfoA and tuaD (sequences shown in SEQ ID NO.9, SEQ ID NO.5-7, respectively) as templates, with primers C6-F / C6-R, kfoC-F / kfoC-R, kfoA-F / kfoA-R, tuaD-F / tuaD-R were respectively amplified by PCR to amplify 4 genes, which were connected to the pGAPZB vector by Gibson assembly to construct pGAPZB-C6ST-T2A-kfoC-T2A2- kfoA-T2A3-tuaD plasmid, wherein T2A, T2A2, T2A3 sequences are designed on the primers.
[0048] (2) To prepare competent cells of Pichia pastoris GS115, the plasmid pGAPZB-C6ST-T2A-kfoC-T2A2-kfoA-T2A3-tuaD obtained above was linearized with fast-cutting enzyme AvrII and then transformed into competent cells. Positive clones were screened on the mycin resistance plate, and the recombinant yeast strain Pp002 integrated with genes C6ST, kfoC, kfoA and tuaD was obtained.
Embodiment 3
[0049] Embodiment 3: Construction of pAO815-ABCI plasmid
[0050] The synthetic gene ABCI (sequence shown in SEQ ID NO.10) was used as a template, and primers ABCI-F / ABCI-R were used for PCR amplification, and one-step cloning enzyme was used to assemble and connect to the pAO815 vector to construct pAO815- ABCI plasmid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com