Preparation method of nicotinamide adenine dinucleotide
A technology of nicotinamide adenine and nicotinamide nucleotide adenosine, which is applied in the field of preparation of nicotinamide adenine dinucleotide, can solve the problems of high concentration and energy consumption, low conversion rate of chemical synthesis, and huge equipment. To achieve the effect of simple and optimized process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
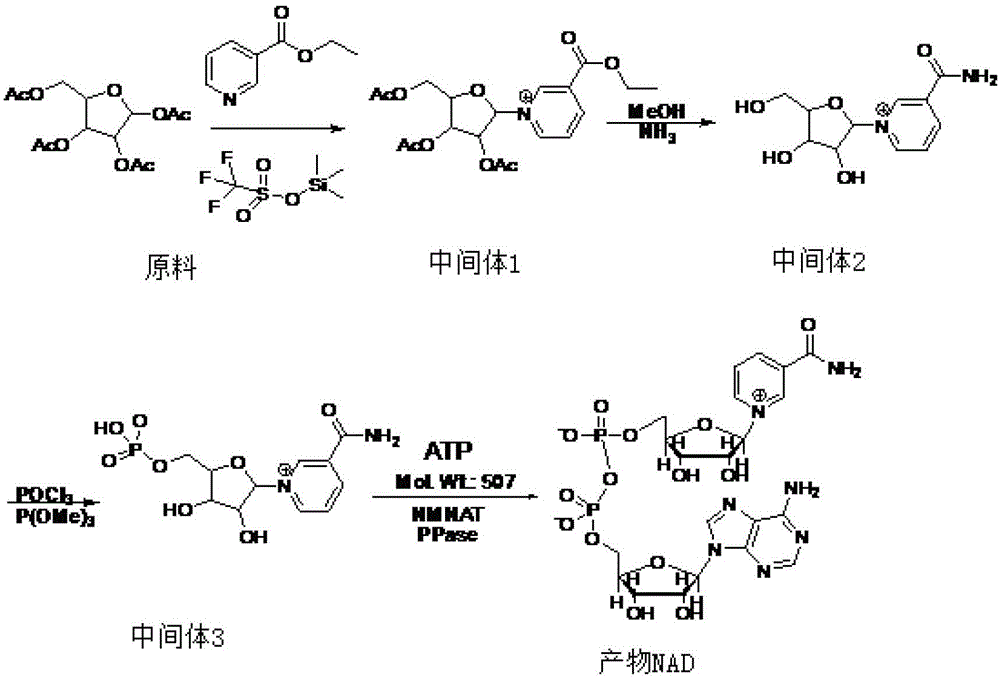
[0031] see figure 1 The preparation and synthesis process roadmap of
[0032] Using 1,2,3,5-tetraacetyl-β-D-ribofuranose as the starting material;
[0033] Step 1: condensation reaction, synthesis such as figure 1 Intermediate 1 shown:
[0034] In a 20L glass reactor, set the stirring and thermometer (range 0-100°C), add 12L of dichloromethane with a triangular funnel, start stirring, and set the stirring speed to 42.5rpm. Throw in 1.2kg (3.77mol) of 1,2,3,5-tetraacetyl-β-D-ribofuranose and stir to dissolve. Open the jacketed circulating water of the reaction kettle (set at 12°C), and when the internal temperature reaches 12°C, add 0.84kg (3.77mol) trimethylsilicone trifluoromethanesulfonate to the dropping funnel, and start to add it slowly, Add 0.84kg (5.65mol) nicotinyl ethyl ester after dropping. The temperature of the circulating water is raised to 50°C, and the internal temperature reaches 48°C, and heat preservation and reflux are started. After reflux for 4h, tak...
Embodiment 2
[0042] Steps one to three are the same as in Example 1.
[0043] Step four:
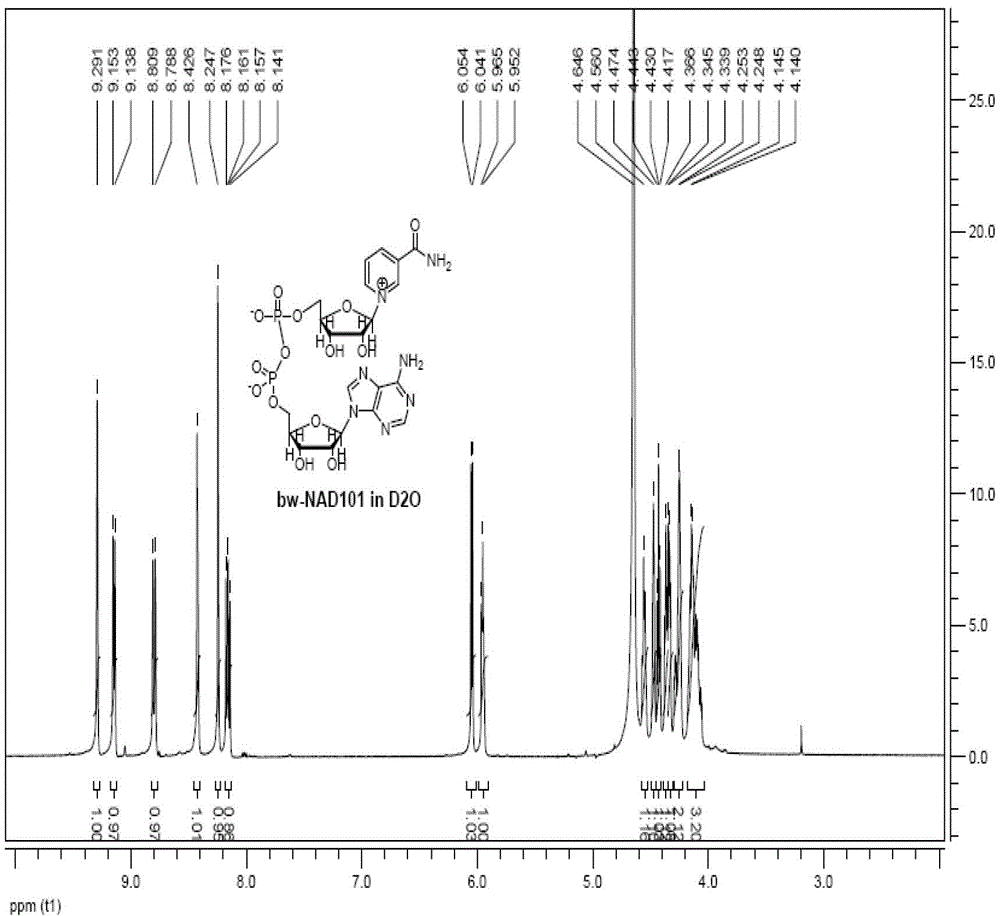
[0044] Set stirring and a thermometer with a range of 0-100°C on the 20L glass reactor, add a buffer solution made of 0.33kg of sodium dihydrogen phosphate, 0.48kg of disodium hydrogen phosphate and 16L of water from the mouth of the kettle with a triangular funnel, and open the reactor. Circulating water (set at 30°C) and stirring, the stirring speed is set at 42.5rpm. Throw 0.78kg (2.32mol) of intermediate 3 nicotinamide mononucleotide and 1.09kg (2.78mol) of adenosine triphosphate. After the materials are completely dissolved, add 0.3kg of whole cells of NMNAT and 0.6kg of whole cells of PPase, and react 10h, sampling. If HPLC shows that the remaining raw materials are ≤ 3% in 4.3 minutes, start to lower the temperature; if HPLC shows that the remaining raw materials are > 3%, continue the reaction until the remaining raw materials are ≤ 3% and then stop the reaction. Blowing, ultrafiltration, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com