Norbornane-2-spiro-cycloalkanone-spiro-2''-norbornane-5,5'',6,6''-tetracarboxylic dianhydride, norbornane-2-spiro-cycloalkanone-spiro-2''-norbornane-5,5'',6,6''-tetracarboxylic acid and ester thereof, method for producing norbornane-2-spiro
A kind of technology of tetracarboxylic dianhydride and norbornane, which can be used in the field of Ming Dynasty and can solve the problems that have not yet been obtained.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0218] Hereinafter, although this invention is demonstrated more concretely based on an Example and a comparative example, this invention is not limited to a following example.
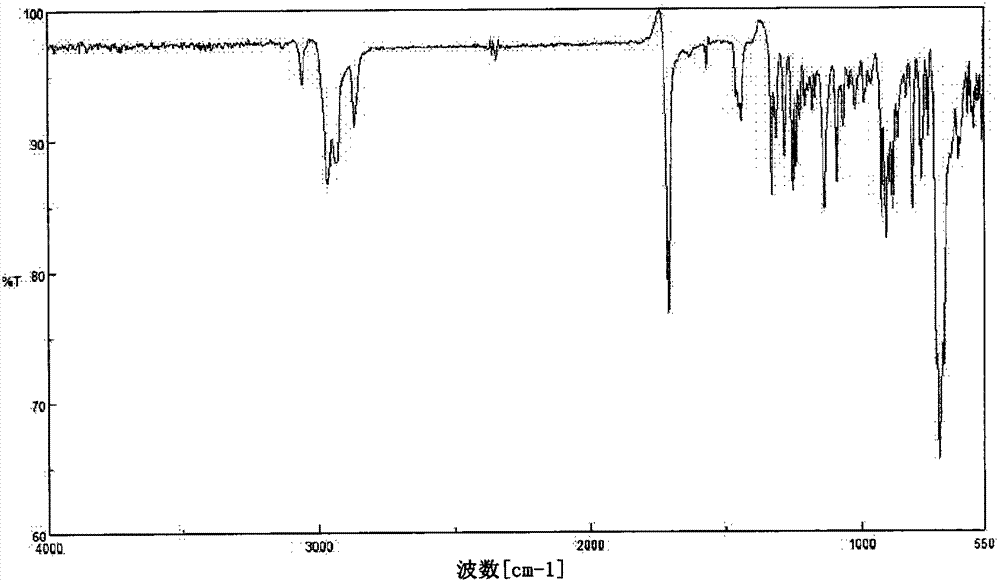
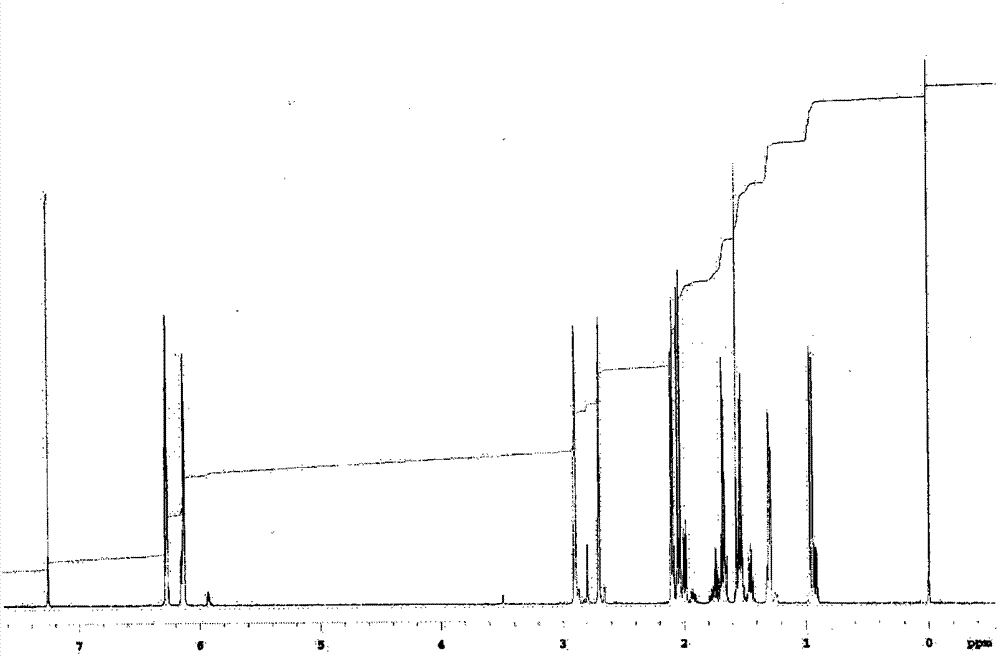
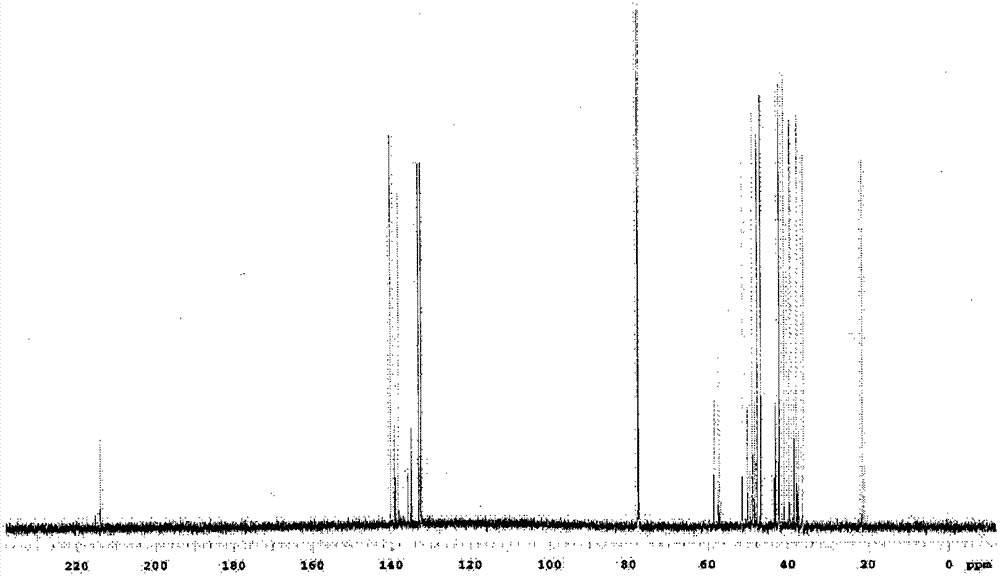
[0219] In addition, in the following, the confirmation of the molecular structure of the compound obtained in each synthesis example and each example is carried out by using an IR detector (manufactured by JASCO Corporation, trade name: FT / IR-460, FT / IR-4100) and NMR A detector (manufactured by VARIAN, trade name: UNITY INOVA-600 and JNM-Lambda500 manufactured by JEOL Ltd.) was used to measure IR and NMR spectra. In addition, the 5% weight loss temperature was measured by heating from room temperature (25°C) at 10°C / min while blowing nitrogen gas using a thermogravimetric analyzer ("TG / DTA220" manufactured by SII NanoTechnology Inc.). The temperature at which the weight of the sample decreases by 5% is obtained. In addition, the glass transition temperature (Tg) is using a differential scanning calor...
Synthetic example 1
[0221] First, 6.83 g (dimethylamine: 75.9 mmol) of the 50 mass % dimethylamine aqueous solution was put into the 100-ml two-necked flask. Next, 8.19 g of 35% by mass aqueous hydrochloric acid (hydrogen chloride: 78.9 mmol) was added to a 100 ml dropping funnel. Next, the above-mentioned dropping funnel was installed on the above-mentioned two-necked flask, and the above-mentioned hydrochloric acid aqueous solution was dropped into the above-mentioned dimethylamine aqueous solution under ice-cooling conditions, and the hydrochloride of dimethylamine was prepared in the above-mentioned two-necked flask. Next, in the above-mentioned two-necked flask, 2.78 g (92.4 mmol) of paraformaldehyde and 2.59 g (30.8 mmol) of cyclopentanone were further added. Next, after attaching a ball head condenser to the above-mentioned two-necked flask, the inside of the above-mentioned two-necked flask was replaced with nitrogen. Afterwards, the above-mentioned two-necked flask was placed in an oil ...
Synthetic example 2
[0227] First, 6.83 g of an aqueous dimethylamine solution (dimethylamine: 75.9 mmol) was added to a 100 ml two-necked flask. Next, 8.19 g of 35% by mass aqueous hydrochloric acid solutions (hydrogen chloride: 78.9 mmol) were added to a 100 ml dropping funnel. Next, the dropping funnel was attached to the two-necked flask, and the aqueous hydrochloric acid solution was dropped into the aqueous dimethylamine solution under ice-cooling to prepare dimethylamine hydrochloride in the two-necked flask. Next, in the above-mentioned two-necked flask, 2.78 g (92.4 mmol) of paraformaldehyde and 3.02 g (30.8 mmol) of cyclohexanone were further added. Next, after attaching a ball head condenser to the above-mentioned two-necked flask, the inside of the above-mentioned two-necked flask was replaced with nitrogen. Afterwards, the above-mentioned two-necked flask was placed in an oil bath of 90° C., and heated and stirred for 4 hours to obtain In the compound n is 3, R 2 , R 3 all are hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com