Synthetic method of 2-mercaptobenzothiazole derivative
A synthesis method and derivative technology, applied in the direction of organic chemistry, etc., can solve problems such as high reaction temperature, complex production process, and difficult control of reaction conditions, and achieve the effects of mild reaction conditions, easy industrialization, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
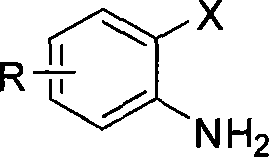
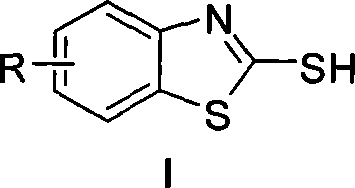
[0018] Embodiment 1, take o-iodoaniline as raw material synthesis 2-mercaptobenzothiazole (with anhydrous ferric chloride as catalyst)
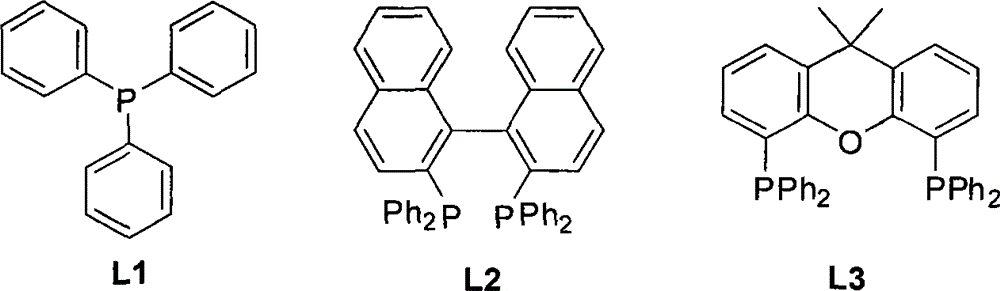
[0019] Add 0.50mmol of o-iodoaniline, 1.50mmol of potassium ethyl xanthate, 0.05mmol of anhydrous ferric chloride and 0.025mmol of 1,1'-binaphthyl-2,2'- For bis-diphenylphosphine (L2), add 2 mL of N, N-dimethylformamide as a reaction solvent under an inert gas environment, and react at 110° C. for 6 hours. After cooling to room temperature, 3 mL of 3N hydrochloric acid was added to the reaction solution and stirred for 2 hours, then 10 mL of saturated sodium bicarbonate solution was added and stirred for 20 minutes. Then the reaction solution was extracted three times with ethyl acetate, the combined organic phases were washed with saturated sodium chloride solution, the organic components were collected, dried over anhydrous magnesium sulfate for 2 hours, after the desiccant was removed, the solvent was distilled off under reduced pressure t...
Embodiment 2
[0020] Embodiment 2, take o-iodoaniline as raw material to synthesize 2-mercaptobenzothiazole (with ferric chloride hexahydrate as catalyst)
[0021] Add 0.54mmol of o-iodoaniline, 1.62mmol of potassium ethyl xanthate, 0.054mmol of ferric chloride hexahydrate and 0.11mmol of 4,5-bisdiphenylphosphine-9,9 into a 50mL three-necked flask -Dimethylxanthene (L3), add 2 mL of N,N-dimethylformamide as a reaction solvent under an inert gas environment, and react at 110° C. for 6 hours. After cooling to room temperature, 3 mL of 3N hydrochloric acid was added to the reaction solution and stirred for 2 hours, then 10 mL of saturated sodium bicarbonate solution was added and stirred for 20 minutes. Then the reaction solution was extracted three times with ethyl acetate, the combined organic phases were washed with saturated sodium chloride solution, the organic components were collected, dried over anhydrous magnesium sulfate for 2 hours, after the desiccant was removed, the solvent was d...
Embodiment 3
[0022] Embodiment 3, take o-iodoaniline as raw material synthesis 2-mercaptobenzothiazole (with iron trifluoride as catalyst)
[0023] Add 0.55mmol of o-iodoaniline, 1.65mmol of potassium ethyl xanthate, 0.055mmol of ferric trifluoride and 0.11mmol of triphenylphosphine (L1) into a 50mL three-necked flask, and then Add 2 mL of N,N-dimethylformamide as a reaction solvent, and react at 110° C. for 6 hours. After cooling to room temperature, 3 mL of 3N hydrochloric acid was added to the reaction solution and stirred for 2 hours, then 10 mL of saturated sodium bicarbonate solution was added and stirred for 20 minutes. Then the reaction solution was extracted three times with ethyl acetate, the combined organic phases were washed with saturated sodium chloride solution, the organic components were collected, dried over anhydrous magnesium sulfate for 2 hours, after the desiccant was removed, the solvent was distilled off under reduced pressure to obtain crude product. The crude p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com