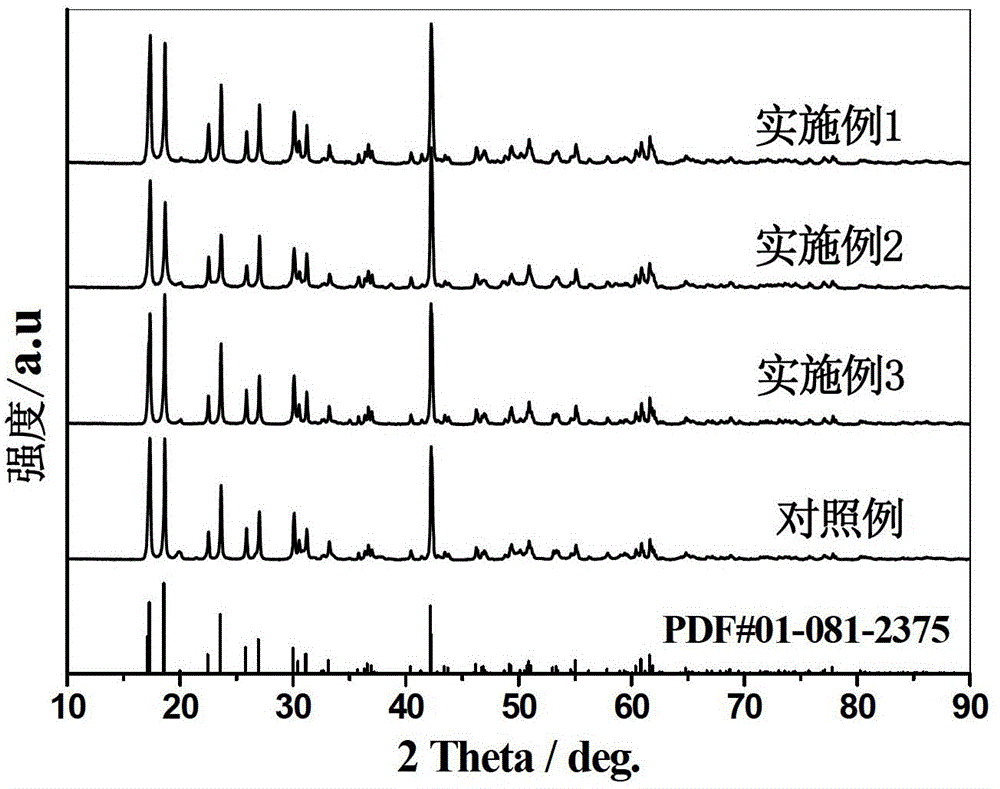
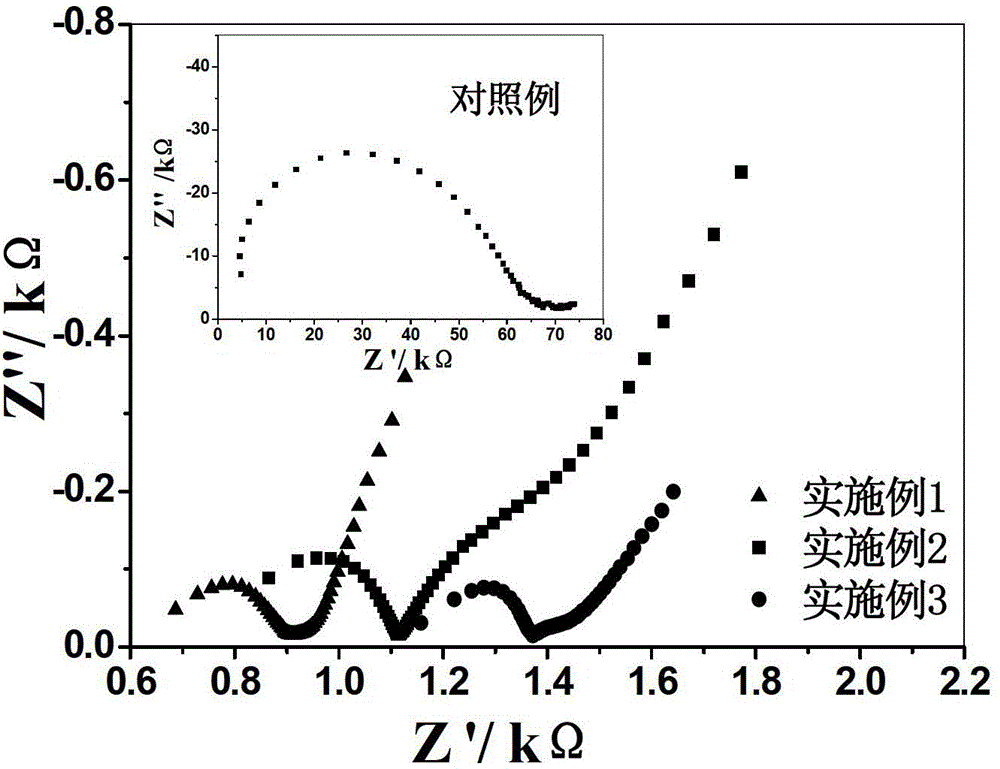
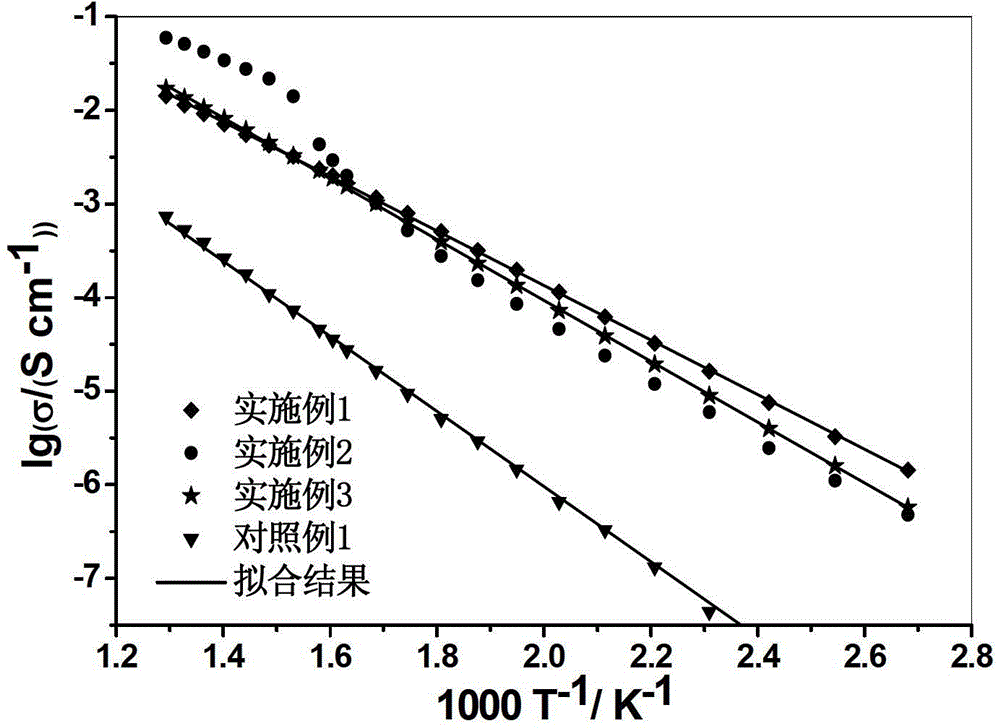
Solid electrolyte and preparation method thereof
A solid electrolyte and crystal technology, applied in chemical instruments and methods, circuits, electrical components, etc., can solve the problems of unsatisfactory solid electrolytes, high boundary resistance of solid particles, chemical stability deviation, etc., and achieve high ionic conductivity and high ionic conductivity. Effects of chemical stability, electrochemical stability improvement, and ionic conductivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) The chemical formula is Li prepared by high-temperature solid-state method 6.15 Zr 1.85 Y 0.15 o 7 Solid electrolyte: LiOH·H 2 O powder, ZrO 2 Powder and Y 2 o 3 The powder was weighed according to the stoichiometric ratio, and it was ground and mixed with an agate mortar for 2 hours. In order to supplement the LiOH·H 2 Loss of lithium ions in O, adding an excess of 10wt% LiOH·H before powder grinding 2 Grinding together with O, in order to obtain a smaller grain boundary resistance between solid particles, adding a mole number of ZrO 2 , Y 2 o 3 and LiOH·H 2 2% of the total moles of O in B 2 o 3 as a sintering aid;
[0048] (2) Put the uniformly mixed powder in step (1) into an alumina crucible with a lid, put the crucible into a muffle furnace at a heating rate of 3°C / min to 800°C and keep it at this temperature for 20 hours, Then the sample is cooled to room temperature at a cooling rate of 10°C / min;
[0049] (3) Grind the white powder obtained in ...
Embodiment 2
[0051] (1) The chemical formula is Li prepared by high-temperature solid-state method 6.15 Zr 1.85 In 0.15 o 7 Solid electrolyte: LiOH·H 2 O powder, ZrO 2 Powder and In 2 o 3 The powder was weighed according to the stoichiometric ratio, and mixed with an agate mortar for 1.5 hours. In order to supplement the LiOH·H 2 Loss of lithium ions in O, adding an excess of 9wt% LiOH·H to the powder before grinding 2 Grinding together with O, in order to obtain a smaller grain boundary resistance between solid particles, adding a mole number of ZrO 2 , Y 2 o 3 and LiOH·H2 2% of the total moles of O in B 2 o 3 as a sintering aid;
[0052] (2) Other steps were processed according to steps (2) and (3) of Example 1 to obtain a solid electrolyte.
Embodiment 3
[0054] (1) The chemical formula is Li prepared by high-temperature solid-state method 6.3 Zr 1.85 Zn 0.15 o 7 Solid electrolyte: LiOH·H 2 O powder, ZrO 2 The powder and ZnO powder were weighed according to the stoichiometric ratio, and mixed with an agate mortar for 2 hours. In order to supplement the LiOH·H 2 Loss of lithium ions in O, adding an excess of 10wt% LiOH·H before powder grinding 2 Grinding together with O, in order to obtain a smaller grain boundary resistance between solid particles, adding a mole number of ZrO 2 , ZnO and LiOH·H 2 2% of the total moles of O in B 2 o 3 as a sintering aid;
[0055] (2) Other steps were processed according to steps (2) and (3) of Example 1 to obtain a solid electrolyte.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com