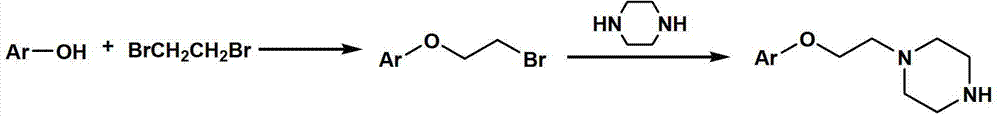
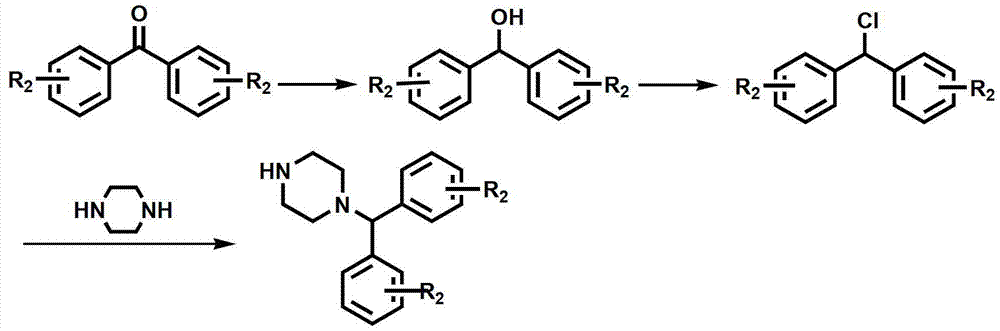
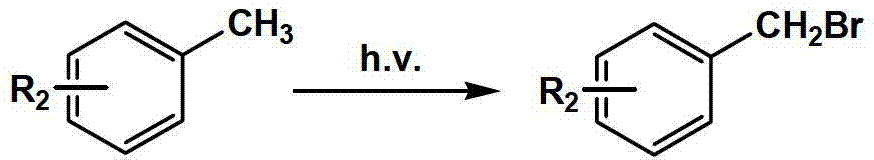
Substituted aryl oxygen ethylpiperazine derivative, preparation method of substituted aryl oxygen ethylpiperazine derivative and application of substituted aryl oxygen ethylpiperazine derivative
A technology of aryloxyethylpiperazine and derivatives is applied in the field of substituted aryloxyethylpiperazine derivatives and their preparation, and the preparation of neuroprotective agents, which can solve the problems of low selectivity, poor oral bioavailability, Difficulty in crossing the blood-brain barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 2-(2-Bromoethoxy)naphthalene
[0042] Add ethyl naphthol (14.4g, 0.10mol), 1,2-dibromoethane (13mL, 0.15mol), water (60mL) into the three-necked flask, stir and raise the temperature to reflux, and add 25%wt of hydroxide Sodium solution (24mL), dripped in about 30 minutes. After reflux for 10 hours, the reaction solution was cooled and separated into layers, and left to stand overnight, a large amount of solids precipitated out. After suction filtration, the filter cake was washed twice with water and recrystallized twice from absolute ethanol to obtain 13.4 g of white crystals with a yield of 54.3%. TLC showed a pure point.
Embodiment 2
[0044] 1-(2-Bromoethoxy)-4-methylbenzene
[0045] Add p-cresol (13g, 0.12mol), 1,2-dibromoethane (15.6mL, 0.18mol), and water (72mL) into a three-necked flask, stir and raise the temperature to reflux, and add 25%wt of hydroxide Sodium solution (28.8mL), dripped in about 45 minutes. After reflux for 10 hours, the reaction liquid was cooled and separated into layers, and stood overnight without solid precipitation. The liquid was separated, the organic layer was washed twice with 40 mL of water, dried over anhydrous magnesium sulfate, filtered with suction, and the organic solvent was distilled off to obtain a pale yellow clear oily liquid. Distilled under reduced pressure to collect a fraction at 108°C / 0.08mmHg, which was initially a clear colorless oily liquid and then solidified into a white solid. Finally, 8.2 g of white solid was obtained, with a yield of 34.2%, and TLC showed a pure point.
Embodiment 3
[0047] 1-(2-Bromoethoxy)-2-methylbenzene
[0048] Add o-cresol (13g, 0.12mol), 1,2-dibromoethane (15.6mL, 0.18mol), and water (72mL) into a three-necked flask, stir and raise the temperature to reflux, and dropwise add 25%wt of hydroxide Sodium solution (28.8mL), dripped in about 45 minutes. After reflux for 13 hours, the reaction liquid was cooled and separated into layers, and stood overnight without solid precipitation. Separate the liquid, dissolve the organic layer with 20mL of dichloromethane, wash with 30mL of 5%wt sodium hydroxide solution for three times, then wash with 30mL of water for three times, dry over anhydrous magnesium sulfate, filter with suction, evaporate the filtrate to remove the solvent, and obtain a light yellow and clear The oily liquid was 10.9g, the yield was 42.3%, and TLC showed a pure point.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com