Asymmetric bismaleimide containing 1, 3, 4-oxadiazole structure and preparation method thereof
The technology of bismaleimide and bismaleamic acid is applied in the field of asymmetric bismaleimide containing 1,3,4-oxadiazole structure and its preparation, and can solve the problem of bismaleimide The problems of poor amine solubility, difficult melt processing, and high melting point can improve the curing process, smooth release, excellent heat resistance and mechanical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
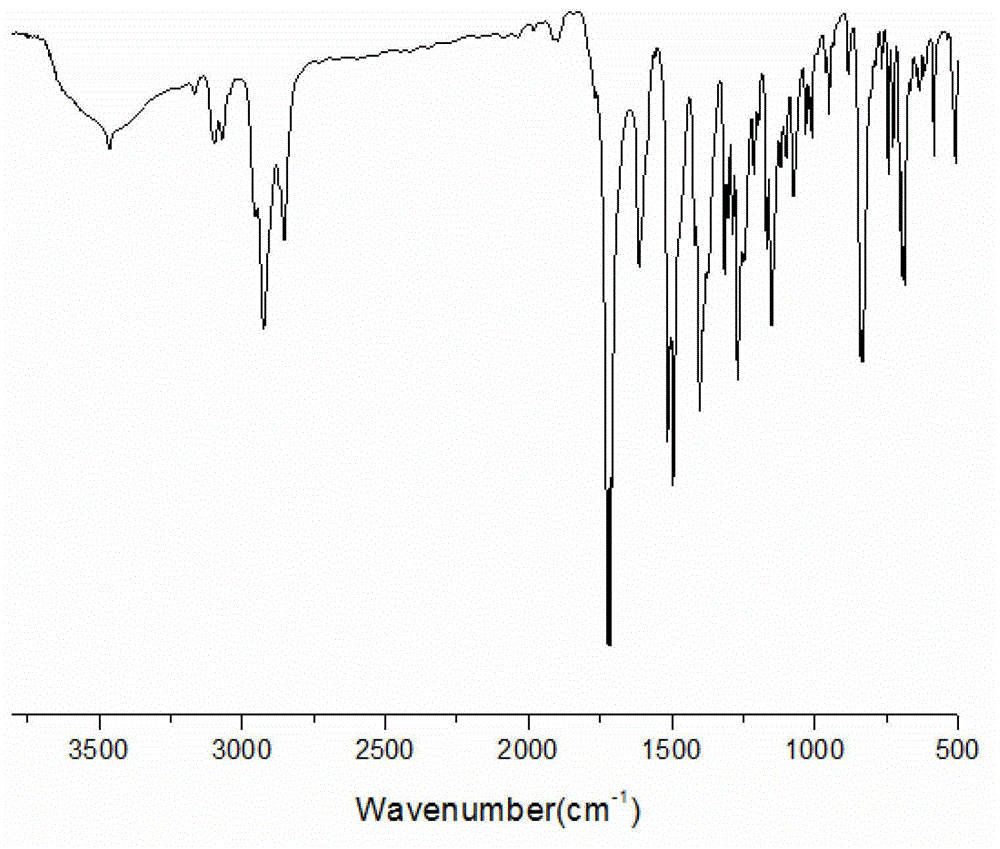
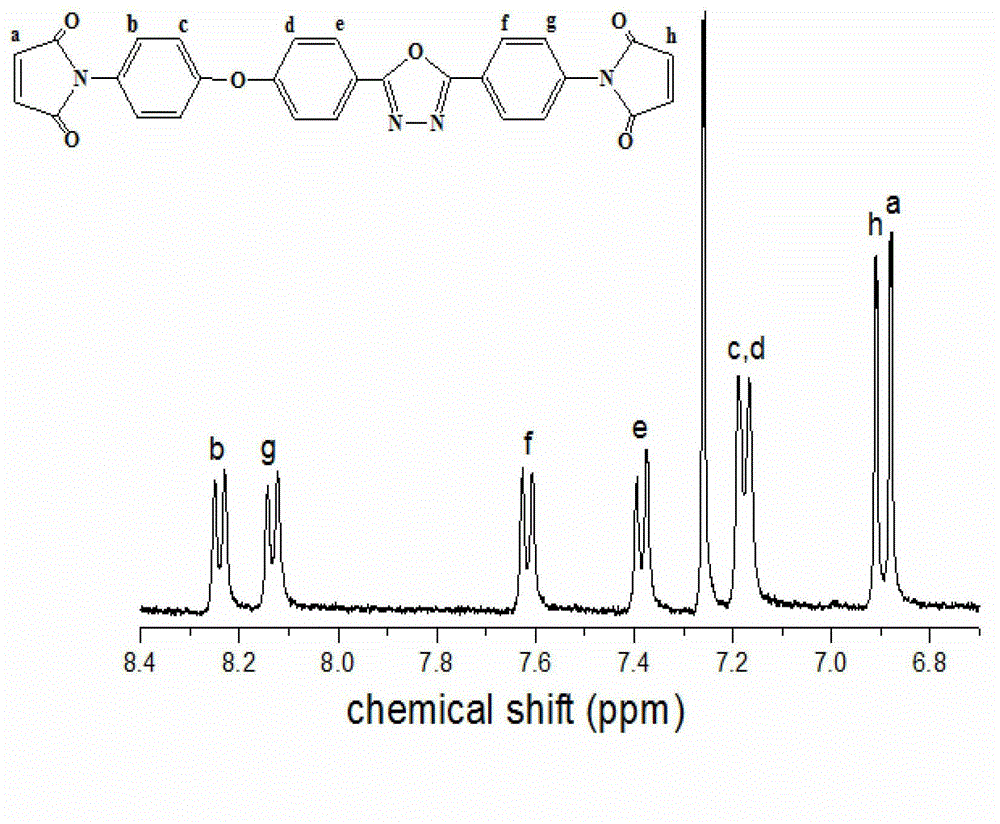
[0023] (A) Dissolve maleic anhydride (0.22mol) in 20ml of N,N-dimethylformamide and stir to dissolve. Dissolve 2-p-aminophenyl-5-(4-p-aminophenoxy)phenyl-1,3,4-oxadiazole (0.1 mol) in 50 ml N,N-dimethylformamide, Slowly drop into the reaction kettle, react at room temperature for 8 hours, after the reaction, pour the reaction solution into deionized water, precipitate a yellow solid, filter with suction, and dry the filter cake under vacuum to obtain 2-bismaleamic acid benzene Base-5-(4-bismaleamidophenoxy)phenyl-1,3,4-oxadiazole. The yield was 93.3%, the purity determined by HPLC was 99.6%, and the melting point was 208°C. FT-I R (KBr, cm -1 ): 3288, 1541 (-NH), 1720, 1631 (C=O).
[0024] (B) Add 2-p-bismaleamic acid phenyl-5-(4-p-bismaleamic acid phenoxy)phenyl-1,3,4-oxadiazole (0.05mol) to the reaction In the kettle, add 300ml of acetone, add 0.2g of sodium acetate, heat to 50°C, add dropwise 8ml of triethylamine and 20ml of acetic anhydride, and react at constant tempe...
Embodiment 2
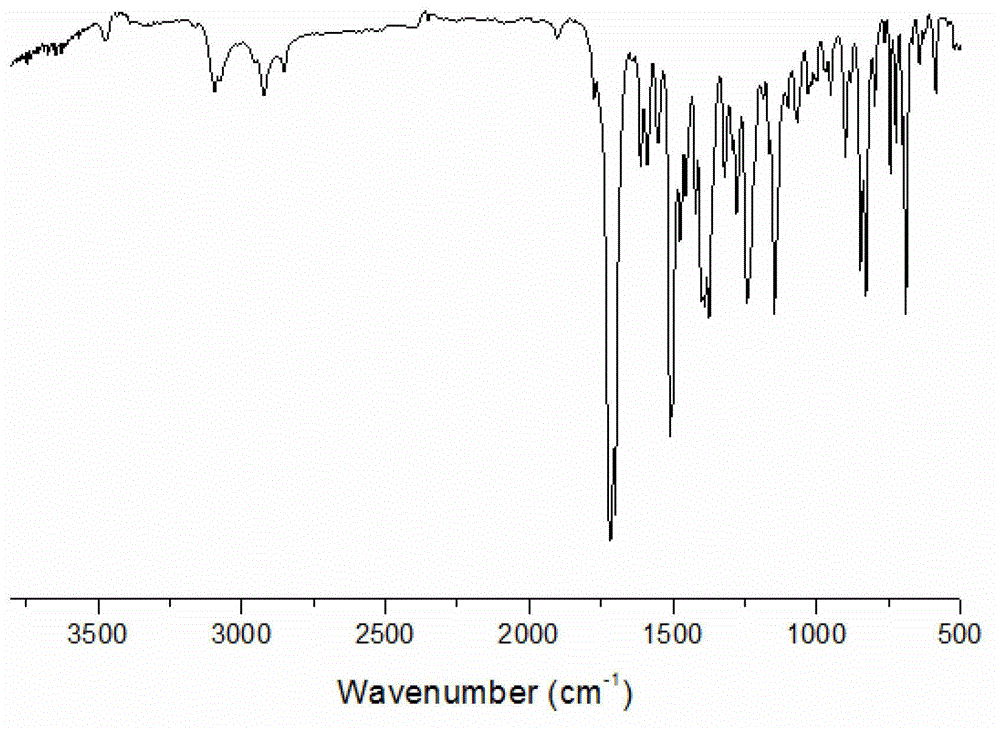
[0026] (A) Dissolve maleic anhydride (0.24mol) in 30ml N,N-dimethylformamide and stir to dissolve. Dissolve 2-p-aminophenyl-5-(3-p-aminophenoxy)phenyl-1,3,4-oxadiazole (0.1 mol) in 40 ml N,N-dimethylformamide, Slowly add it dropwise into the reaction kettle, and react at room temperature for 10 hours. After the reaction, pour the reaction solution into deionized water to precipitate a yellow solid, filter it with suction, and dry the filter cake in vacuum to obtain 2-bismaleamic acid benzene Base-5-(3-bismaleamidophenoxy)phenyl-1,3,4-oxadiazole. The yield was 91.3%, the purity determined by HPLC was 99.0%, and the melting point was 184°C. FT-IR (KBr, cm -1 ): 3288, 1551 (-NH), 1719, 1626 (C=O).
[0027] (B) Add 2-p-bismaleamic acid phenyl-5-(3-p-bismaleamic acid phenoxy)phenyl-1,3,4-oxadiazole (0.05mol) In the reaction kettle, add 200ml of acetone, add 0.18g of cobalt acetate, heat to 60°C, add 10ml of triethylamine and 25ml of acetic anhydride dropwise, and react at const...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com