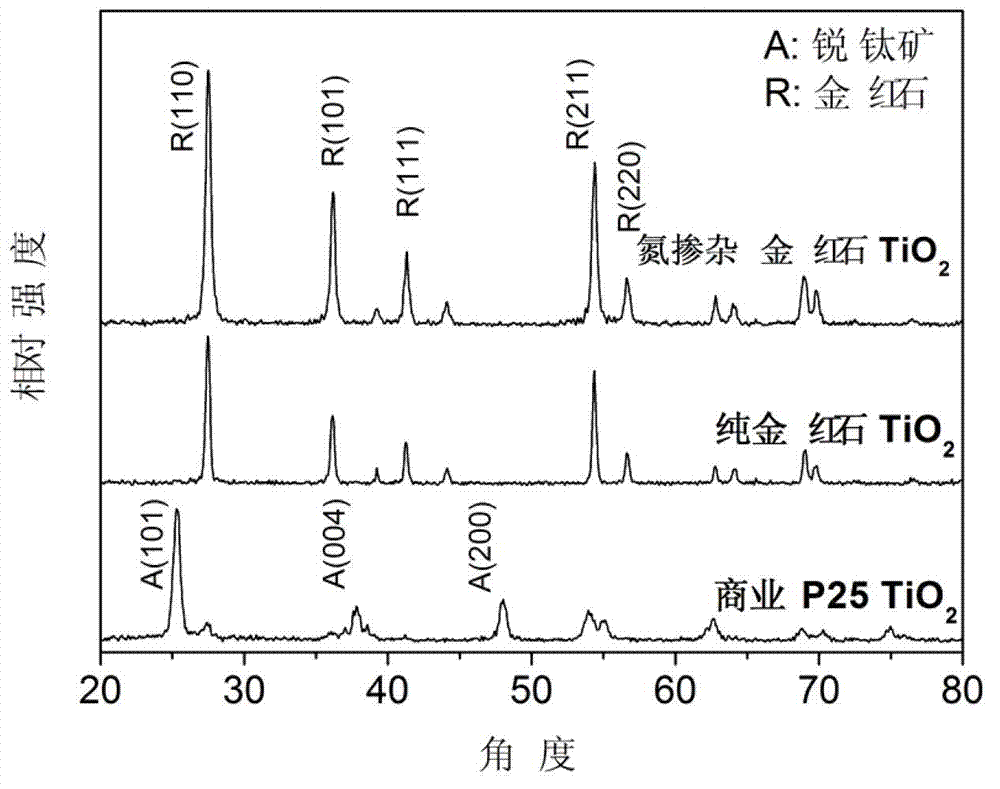
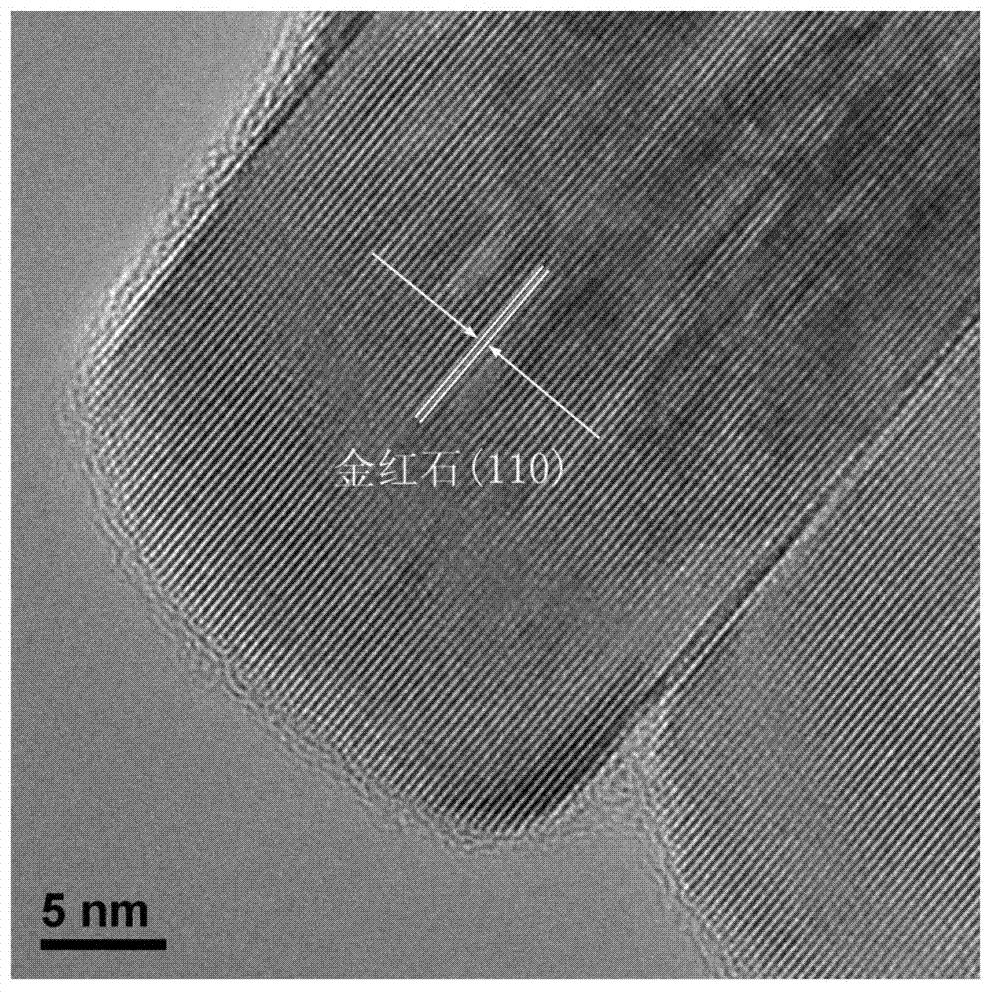
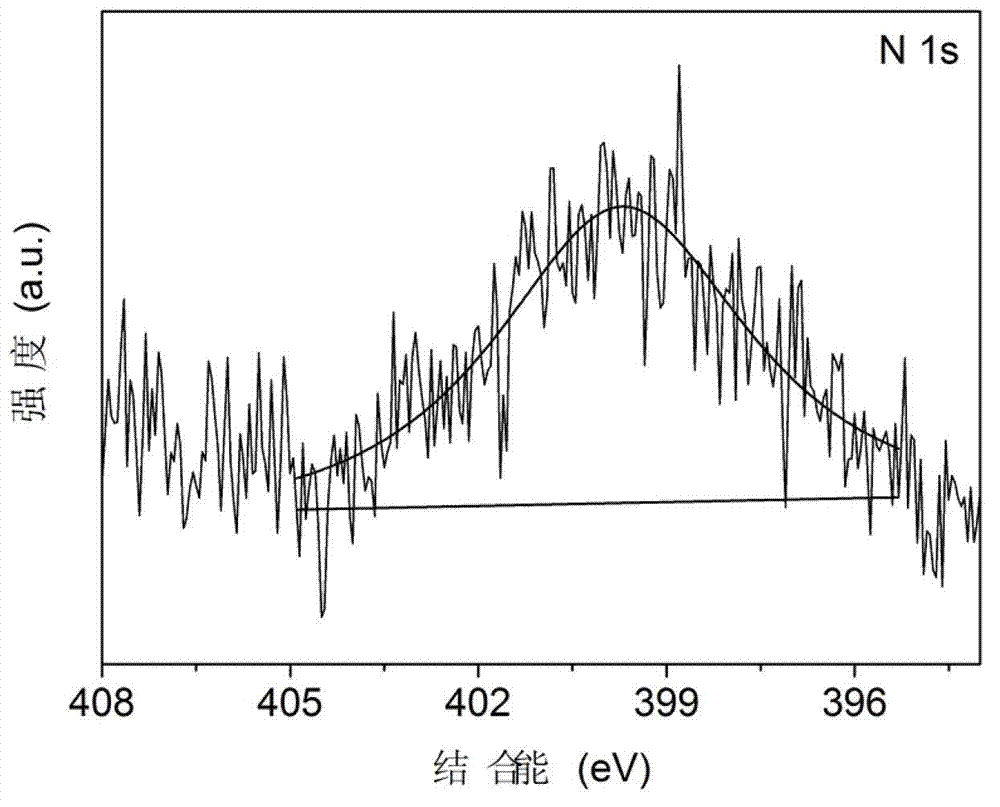
Method for preparing nitrogen-doped rutile TiO2 selective photocatalyst
A photocatalyst and nitrogen-doped technology, which is applied in the direction of light water/sewage treatment, oxidized water/sewage treatment, textile industry wastewater treatment, etc. It can solve the problems that cannot pass through, organic molecules are difficult to achieve selectivity, and cannot be degraded. , to achieve the effect of preferential and selective catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 0.39Kg titanium nitride and 4L concentration of 10M HNO 3 1. Mix 1 L of HCl with a concentration of 10M to form a precursor, and place it in a hydrothermal reaction kettle at a constant temperature of 180°C for 24 hours; perform solid-liquid centrifugation on the solid-liquid mixture after the hydrothermal reaction, pour out the liquid product, and take out the solid product , washed with deionized water three times and then dried at 80°C for 10 hours; the dried product was sintered at 600°C for 90 minutes; the sintered product was ground into powder to obtain nitrogen-doped rutile TiO 2 nanorod.
Embodiment 2
[0028] 0.39Kg titanium nitride and 5L concentration of 10M HNO 3 1. Mix 1 L of HCl with a concentration of 10M to form a precursor, and place it in a hydrothermal reaction kettle at a constant temperature of 180°C for 27 hours; perform solid-liquid centrifugation on the solid-liquid mixture after the hydrothermal reaction, pour out the liquid product, and take out the solid product , washed with deionized water three times and then dried at 80°C for 13 hours; the dried product was sintered at 600°C for 100 minutes; the sintered product was ground into powder to obtain nitrogen-doped rutile TiO 2 nanorod.
Embodiment 3
[0030] 0.39Kg titanium nitride and 5L concentration of 10M HNO 3 2. Mix 2L of HCl with a concentration of 10M to form a precursor, and place it in a hydrothermal reaction kettle at a constant temperature of 190°C for 30 hours; perform solid-liquid centrifugation on the solid-liquid mixture after the hydrothermal reaction, pour out the liquid product, and take out the solid product , washed 4 times with deionized water and dried at 90°C for 16 hours; the dried product was sintered at 700°C for 100 minutes; the sintered product was ground into powder to obtain nitrogen-doped rutile TiO 2 nanorod.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com