Method for high selectively synthesizing dimethylbenzene through benzene and methanol alkylation reaction
An alkylation reaction, high selectivity technology, applied in organic chemistry, bulk chemical production, condensation between hydrocarbons and non-hydrocarbons, etc., can solve the low selectivity of xylene, no improvement in conversion rate, stability Deletion and other problems can be achieved to enhance reaction stability and inhibit reaction coking and inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
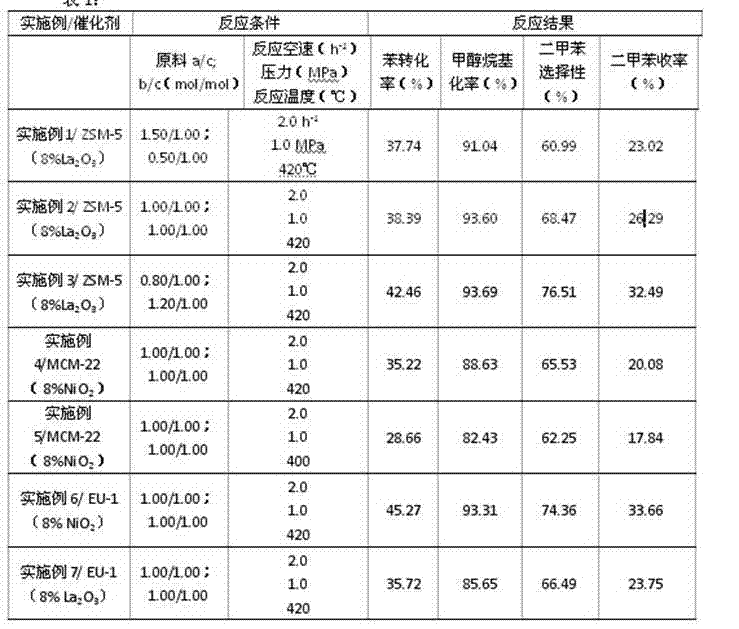
[0023] Example 1: Put an appropriate amount of glass beads at both ends of the stainless steel fixed bed reaction tube of 1.5cm in the inner diameter, and a 4.00g cylindrical catalyst ZSM-5 (8%LA (8%LA (8%LA 2 O 3 ) (Si / Al = 24), and then connect with hydrogen gas, H 2 The Moore of the hydrocarbon is 2.0: 1 to ensure that the airflow is uniformly flowing over the catalyst bed layer; the reaction pressure is 1.0MPa, and it is heated to the reaction set temperature of 420 ° C by 100MINS.) = 1.50 / 1.00 (mol / mol), B (toluene) / C (methanol) = 0.50 / 1.00 (mol / mol), the quality of the quality is 2.0H -1 , Examine the catalytic effect, analyze the chromatography of the sample, calculate the one -way conversion rate of the benzene after the reaction, the methanol alkyrization rate, the selectability of dyshane, and the second tailoris.
Embodiment 2
[0024] Example 2: The difference between this embodiment and Example 1 is that the raw material group becomes A (benzene) / C (methan) = 1.00 / 1.00 (mol / mol), B (methane) / C (methanol) = 1.00 / 1.00(Mol / mol); reactive gas is CO 2 , And CO 2 The Moorby with hydrocarbons is 1.0: 1; other conditions are shown in Table 1.
Embodiment 3
[0025] Example 3: The difference between this embodiment and Example 1 is that the raw material group becomes A (benzene) / C (methan) = 0.80 / 1.00 (mol / mol), B (methane) / C (methanol) = 1.20 / 1.00(Mol / mol); 2 , And n 2 The Moorby with raw materials is 3.0: 1; other conditions are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com