Method for producing nitric oxide and synthesizing dimethyl oxalate through carbonylation
A technology of dimethyl oxalate and nitric oxide, which is applied in the direction of carbon monoxide or formate reaction preparation, nitric oxide, nitrogen oxides/oxyacids, etc., can solve the problems of production and storage safety hazards, and achieve low cost Low, good market value, effect of equipment operation safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
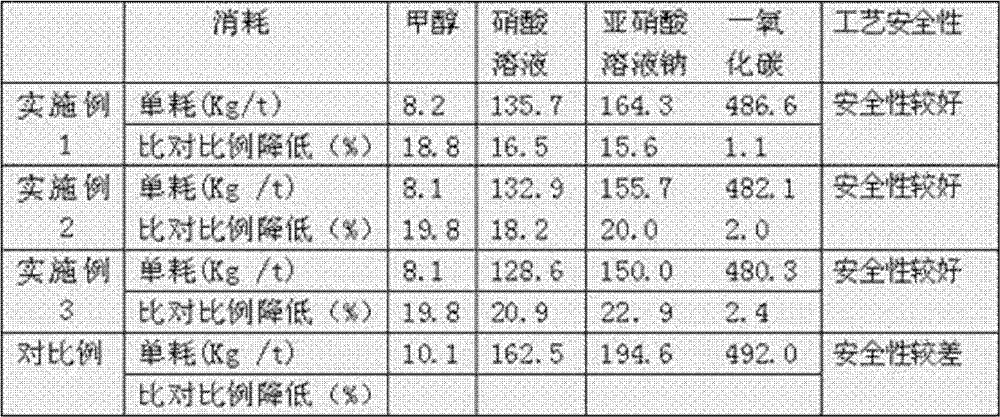
[0029] Embodiment 1: annual production scale of 2000 tons of dimethyl oxalate
[0030] The specifications of the reaction raw materials are as follows:
[0031] The concentration of dilute nitric acid is 50%, the concentration of sodium nitrite solution is 45%, the purity of CO is 98.5%, the purity of methanol is 99.8%, and the purity of oxygen is 99.5%.
[0032] The reaction feed is as follows:
[0033] CO: 109Nm 3 / h,
[0034] o 2 : 27Nm 3 / h,
[0035] Methanol: 770Kg / h,
[0036] Nitric acid: 38Kg / h,
[0037] Sodium nitrite: 46Kg / h.
[0038] The main control indicators of carbonylation reaction are as follows:
[0039] Into the reactor gas components: NO 2.5%, CO 15%, methyl nitrite 10%, methanol 15%, the rest is N 2 .
[0040] Reaction pressure: 0.3MPa.
[0041] Reaction temperature: 140°C.
[0042] Products and methanol consumption are as follows:
[0043] The amount of dimethyl oxalate in the product is: 280K g / h,
[0044] Dimethyl oxalate purity: ≥99.5%, ...
Embodiment 2
[0047] Embodiment 2: annual production scale of 5000 tons of dimethyl oxalate
[0048] The specifications of the reaction raw materials are as follows:
[0049] The concentration of dilute nitric acid is 50%, the concentration of sodium nitrite solution is 45%, the purity of CO is 98.5%, the purity of methanol is 99.8%, and the purity of oxygen is 99.5%.
[0050] The reaction feed is as follows:
[0051] CO: 270Nm 3 / h,
[0052] o 2 : 67.5Nm 3 / h,
[0053] Methanol: 1850Kg / h,
[0054] Nitric acid: 93Kg / h,
[0055] Sodium nitrite: 109Kg / h.
[0056] The main control indicators of carbonylation reaction are as follows:
[0057] Into the reactor gas components: NO 3.0%, CO 18%, methyl nitrite 10%, methanol 16%, the rest is N 2 .
[0058] Reaction pressure: 0.4MPa.
[0059] Reaction temperature: 140°C.
[0060] Products and methanol consumption are as follows:
[0061] The amount of dimethyl oxalate in the product is: 700K g / h,
[0062] Dimethyl oxalate purity: ≥99.5%,...
Embodiment 3
[0065] Embodiment 3: annual production scale of 10000 tons of dimethyl oxalate
[0066] The specifications of the reaction raw materials are as follows:
[0067] The concentration of dilute nitric acid is 50%, the concentration of sodium nitrite solution is 45%, the purity of CO is 98.5%, the purity of methanol is 99.8%, and the purity of oxygen is 99.5%.
[0068] The reaction feed is as follows:
[0069] CO: 561Nm 3 / h,
[0070] o 2 : 138Nm 3 / h,
[0071] Methanol: 3980Kg / h,
[0072] Nitric acid: 92Kg / h,
[0073] Sodium nitrite: 370Kg / h.
[0074] The main control indicators of carbonylation reaction are as follows:
[0075] Into the reactor gas components: NO 2.0%, CO 15%, methyl nitrite 12%, methanol 15%, the rest is N 2 .
[0076] Reaction pressure: 0.35MPa.
[0077] Reaction temperature: 135°C.
[0078] Products and methanol consumption are as follows:
[0079] The amount of dimethyl oxalate in the product is: 1460K g / h,
[0080] Dimethyl oxalate purity: ≥99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com