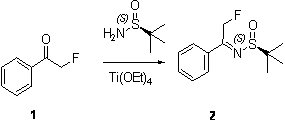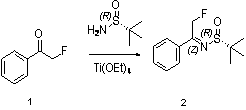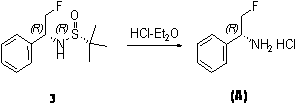Preparation method of chiral 2-fluoromethyl phenyl ethylamine
A technology of fluoromethylphenylethylamine and fluoromethyl, which is applied in the field of preparation of chiral 2-fluoromethylphenylethylamine, can solve the problems of high cost, complex synthesis method, long synthesis route and the like, and achieves simple and convenient EFFECT OF EFFECTIVE SYNTHETIC ROUTES
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: Preparation of (R)-2-fluoromethylphenylethylamine
[0016] 1: Preparation of (2-fluoromethyl-1-phenyl-ethyl)-(R)-tert-butylsulfinimide
[0017] Reaction formula:
[0018]
[0019] Steps:
[0020] Get a 1000ml round-bottomed flask and put it into a magnetic stirrer and install a reflux condenser, add 2-fluoro-1-phenylethanone (80 g, 579 mmol), (R)-tert-butylsulfinamide ( 80 g, 600 mmol ), tetraethyl titanate (160 g, 700 mmol) and 200 ml tetrahydrofuran. The reaction mixture was stirred at room temperature under nitrogen protection for 15 hours, the reaction solution was poured into 1L of water, extracted with ethyl acetate, and the organic phase was concentrated under reduced pressure to obtain (2-fluoromethyl-1-phenyl-ethyl)-( R)-tert-butylsulfinimide crude product (98 g), this crude product was directly used in the next reaction.
[0021] 2: Preparation of (R)-(2-fluoromethyl-1-phenyl-ethyl)-(R)-tert-butylsulfinamide
[0022] Reaction formula: ...
Embodiment 2
[0033] Embodiment 2: Preparation of (S)-2-fluoromethylphenylethylamine
[0034] 1: Preparation of (2-fluoromethyl-1-phenyl-ethyl)-(S)-tert-butylsulfinimide
[0035] Reaction formula:
[0036]
[0037] Steps:
[0038] Get a 1000ml round-bottomed flask and put it into a magnetic stirrer and install a reflux condenser, add 2-fluoro-1-phenylethanone (50 g, 362 mmol), (S)-tert-butylsulfinamide ( 50 g, 376 mmol ), tetraethyl titanate (100 g, 438 mmol) and 200 ml tetrahydrofuran. The reaction mixture was stirred at room temperature under nitrogen protection for 15 hours, the reaction solution was poured into 1L of water, extracted with ethyl acetate, and the organic phase was concentrated under reduced pressure to obtain (2-fluoromethyl-1-phenyl-ethyl)-( S)-tert-butylsulfinimide (65 g, 75%), this crude product was directly used in the next reaction.
[0039] 2: Preparation of (S)-(2-fluoromethyl-1-phenyl-ethyl)-(S)-tert-butylsulfinamide
[0040] Reaction formula:
[0041] ...
Embodiment 3
[0051] Embodiment 3: Preparation of (R)-2-fluoromethylphenylethylamine
[0052] 1: Preparation of (2-fluoromethyl-1-phenyl-ethyl)-(R)-tert-butylsulfinimide
[0053] Reaction formula:
[0054]
[0055] Steps:
[0056] Get a 100ml round-bottomed flask and put it into a magnetic stirrer and install a reflux condenser, add 2-fluoro-1-phenylethanone (5 g, 36 mmol), (R)-tert-butylsulfinamide ( 5 g, 41 mmol ), tetraisopropyl titanate (14.2 g, 50 mmol) and 50 ml tetrahydrofuran. This reaction mixture was stirred at room temperature under nitrogen protection for 15 hours, this reaction solution was poured into 100mL water, extracted with ethyl acetate, and the organic phase was concentrated under reduced pressure to obtain (2-fluoromethyl-1-phenyl-ethyl)-( R)-tert-butylsulfinimide crude product (10 g), this crude product was directly used in the next reaction.
[0057] 2: Preparation of (R)-(2-fluoromethyl-1-phenyl-ethyl)-(R)-tert-butylsulfinamide
[0058] Reaction formula: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com