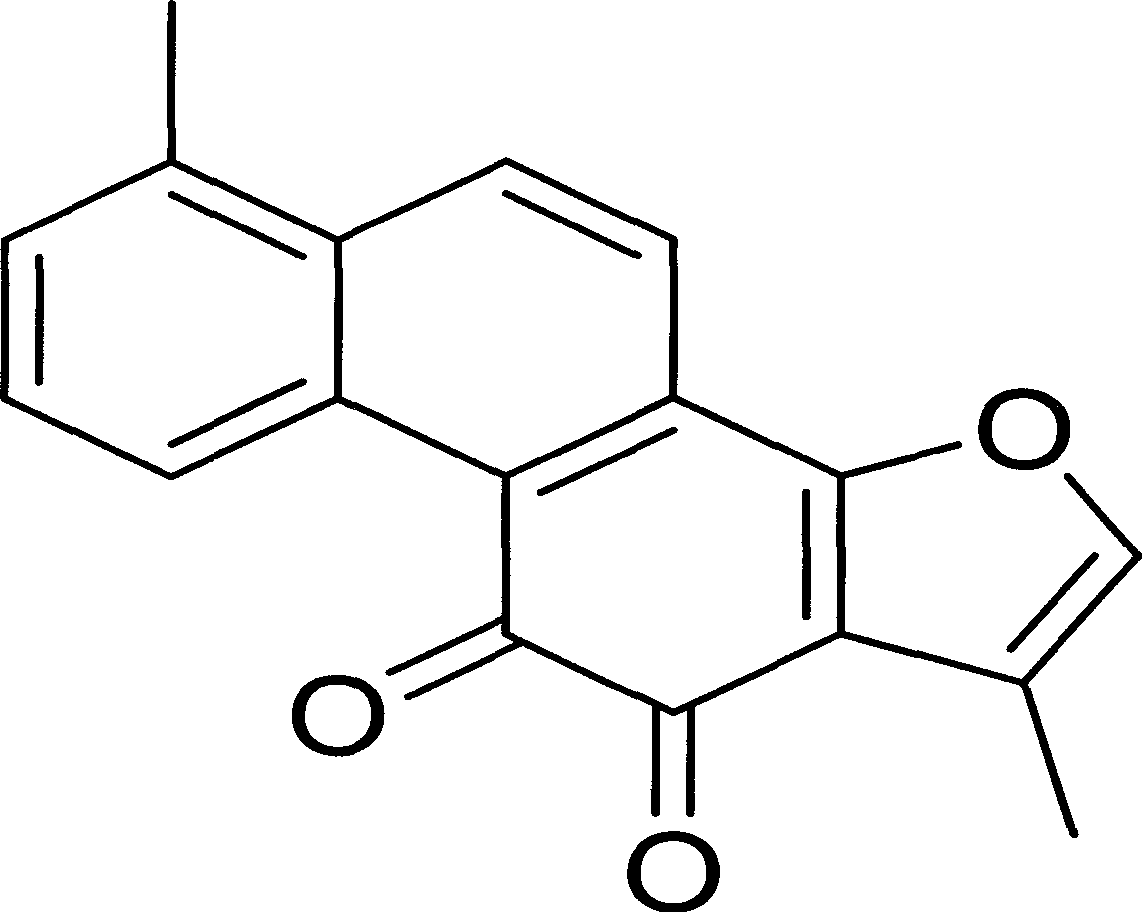
Application of tanshinone I in preparation of medicine for treating psoriasis
A technology for tanshinone and psoriasis is applied in the application field of tanshinone I in the preparation of medicines for the treatment of psoriasis, which can solve the problems of large toxic and side effects, intolerance of patients, expensive biological preparations and the like, and achieves strong development prospects and safety. Good sex, significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Prepare oral Tanshinone I capsules (1000 capsules) for the treatment of psoriasis. The weight of the components is as follows:
[0017]
[0018] Production process: Mix Tanshinone I and lactose evenly, moisten it with water, then pass through a 20-mesh sieve, dry, and again pass through a 22-mesh sieve, then add magnesium stearate, mix well, and fill the capsule.
Embodiment 2
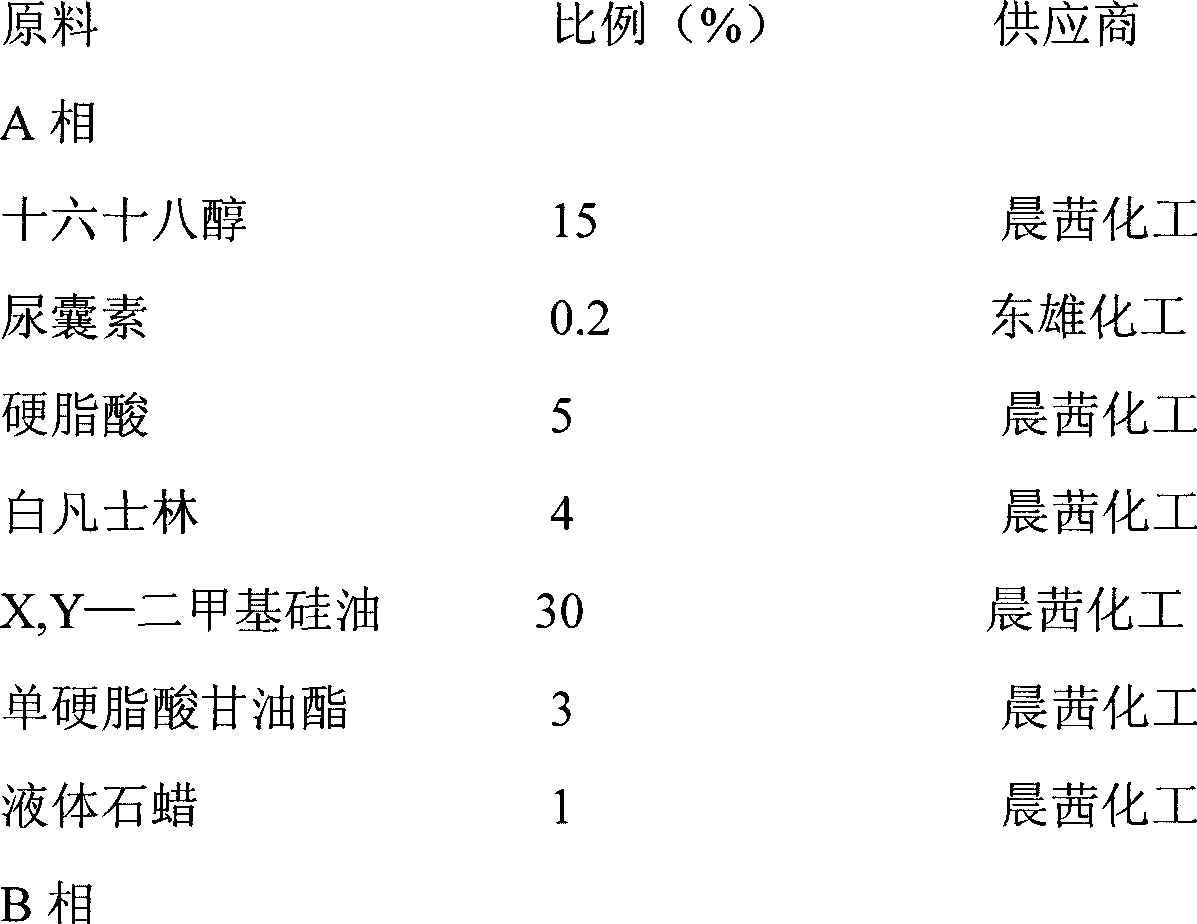
[0020] To prepare the tanshinone I cream for external use in the treatment of psoriasis, the weight percentages of the components are as follows:
[0021]
[0022]
[0023] Production Process:
[0024] 1. Weigh all the components of phase A and mix at 90℃;
[0025] 2. Weigh all the components of phase B and mix them at 90℃;
[0026] 3. Slowly add phase A to phase B, stirring while adding, for about 20 minutes;
[0027] 4. After homogenizing for 20 minutes, cool to room temperature to obtain the cream.
Embodiment 3
[0029] Experimental research report of Tanshinone I inhibiting the proliferation of human keratinocytes (HaCaT cells):
[0030] 1. Materials and methods
[0031] 1.1 Material Tanshinone I standard product was purchased from the National Institute for the Control of Pharmaceutical and Biological Products; the positive control drug anthranol was purchased from Wuhan Tongxing Biotechnology Company; 3-(4,5-dimethylthiazole-2)-2,5-two Phenyltetrazolium bromide (MTT) was purchased from Beijing Dingguo Biotechnology Co., Ltd.; Hoechest 33258 fluorescent dye was purchased from sigma, USA; Annexin V cell apoptosis detection kit was purchased from Lianke Biotechnology Co., Ltd.
[0032] 1.2 Instruments BX-61 fluorescence microscope from Olympus, Japan; FC-500 flow cytometer from Beckman Coulter, USA; Epoch microplate reader from BIOTEK, USA.
[0033] 1.3 method
[0034] 1.3.1 MTT detects cell proliferation and IC 50 Calculation
[0035] Choose logarithmic growth phase HaCaT cells, digest with dig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com