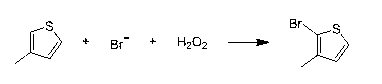Synthesis method of 3-methylthiophene-2-aldehyde
A technology of methyl thiophene and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of difficult separation of isomers, practical value of waste water, and difficult availability of raw materials, and achieve the effects of cost reduction, easy product purification, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthetic method of the 3-methylthiophene-2-carboxaldehyde of the present embodiment comprises the following steps successively:
[0020] The first step is to synthesize 3-methyl-2-bromothiophene: the three-necked flask is equipped with mechanical stirring, a thermometer, and a dropping funnel, and 98.17 g (1 mole) of 3-methylthiophene and 48% hydrogen bromide are added to the three-necked flask 202g (1.2 mole) of acid, cooled to 0°C, then control the temperature at 5-10°C and add 113.4g (1 mole) of hydrogen peroxide with a concentration of 30% dropwise, after the dropwise addition, raise the temperature to 20°C, stir for 2 hours and then let stand. Liquid separation, the organic layer was washed with sodium sulfite aqueous solution and sodium carbonate aqueous solution, and rectified under reduced pressure to obtain 165 g of 3-methyl-2-bromothiophene with a purity of 99.3% and a yield of 93.1%;
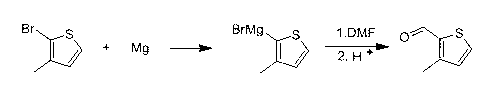
[0021] The second step is to synthesize 3-methylthiophene-2-carbaldeh...
Embodiment 2
[0023] The synthetic method of the 3-methylthiophene-2-carboxaldehyde of the present embodiment comprises the following steps successively:
[0024] The first step is to synthesize 3-methyl-2-bromothiophene: a three-necked flask is equipped with mechanical stirring, a thermometer, and a dropping funnel, and 98.17 g (1 mole) of 3-methylthiophene and 1.2 mole (with Bromide ion meter), cooled to 0°C, then controlled the temperature at 5-10°C, added dropwise 113.4g (1 mole) of hydrogen peroxide with a concentration of 30%, raised the temperature to 20°C after the dropwise addition, stirred for 2 hours, let stand, and separated the liquid , the organic layer was washed with aqueous sodium sulfite and aqueous sodium carbonate, and rectified under reduced pressure to obtain 162 g of 3-methyl-2-bromothiophene with a purity of 99.2% and a yield of 91.49%;
[0025] The second step is to synthesize 3-methylthiophene-2-carbaldehyde: add 25.5g (1.05mole) of magnesium chips, 20ml of tetrahy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com