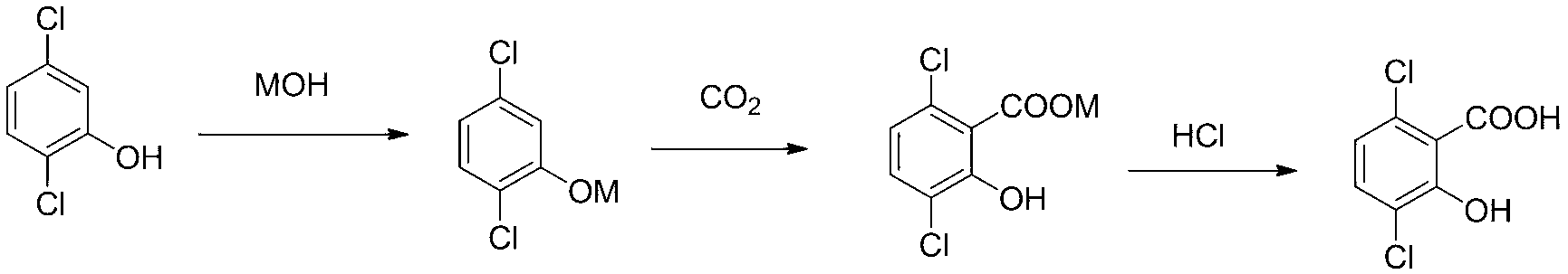
Synthetic method for 3,6-dichloro-2-hydroxybenzoic acid
A technology of hydroxybenzoic acid and hydroxybenzoate, which is applied in the field of synthesis of 3,6-dichloro-2-hydroxybenzoic acid, can solve the problems of difficult realization and high requirements, and reduce reaction cost and production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, a kind of synthetic method of 3,6-dichloro-2-hydroxybenzoic acid, carries out following steps successively:
[0028] 1) Dissolve 73g (0.415mol) 2,5-dichlorophenol (95% purity) in 24.8g (0.381mol) KOH (86% purity) and 40ml water in a 1000ml three-necked flask, then add Mix 480ml of xylene as a solvent evenly, heat up to 142~145°C, dehydrate with a water separator until almost no water is evaporated, and obtain 2,5-dichlorophenoxide potassium solution.
[0029] 2) Transfer the potassium 2,5-dichlorophenate solution obtained in the above step 1) to the autoclave, then add 32g of potassium chloride, and fill the autoclave with CO 2 To 3Mpa, after being stabilized, empty the air, and continue the same operation for 3 times, so as to fully replace the air in the kettle; then fill with CO 2 to 5Mpa , and heating; finally, control the reaction temperature to 145° C. and the reaction pressure to 6 Mpa to carry out the carboxylation reaction, and the reaction time...
Embodiment 2
[0031] Embodiment 2, a kind of synthetic method of 3,6-dichloro-2-hydroxybenzoic acid, carries out following steps successively:
[0032] 1) Dissolve 146g (0.83mmol) 2,5-dichlorophenol (95%) in 98gKOH (0.84mol) aqueous solution (48% concentration) in a 1000ml three-necked flask, and then add 640ml xylene as Mix the solvent evenly, heat up to 142~145°C, dehydrate with a water separator until almost no water evaporates, and obtain 2,5-dichlorophenoxide potassium solution.
[0033] 2) Transfer the potassium 2,5-dichlorophenate solution obtained in the above step 1) to the autoclave, then add 63g of potassium chloride, and use CO 2 After fully replacing the air in the kettle, carry out the carboxylation reaction. The controlled temperature is 145°C, the pressure is 6Mpa, and the reaction time is 12h. After the reaction, a reaction product containing potassium 3,6-dichloro-2-hydroxybenzoate was obtained.
[0034] 3) After cooling the reaction product containing potassium 3,6-dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com