Method for preparing copper (zinc) acrylate resin
A technology of polyacrylic acid resin and polyacrylic acid copper, which is applied in the synthesis of copper acrylate and zinc acrylate resin, the manufacture of self-polishing antifouling coatings, and the synthesis of resin materials, which can solve problems such as unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
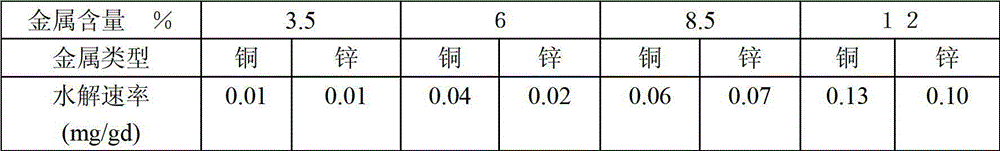
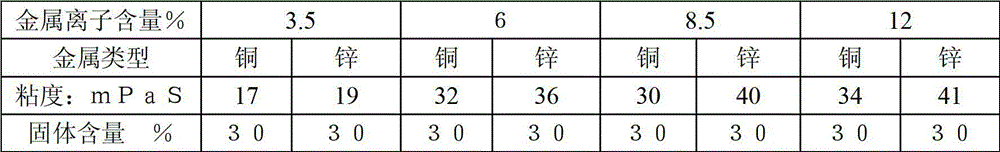
Image
Examples
Embodiment 1
[0056] Synthesis of No.1 Polyacrylic Resin
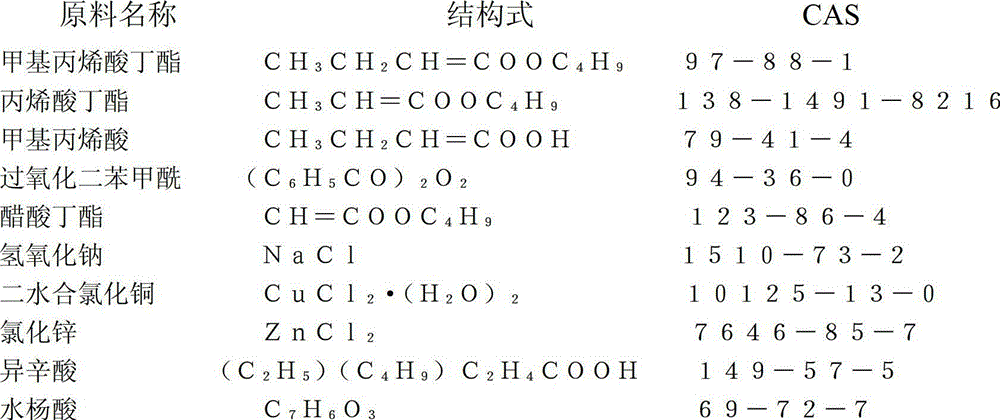
[0057] Raw materials (parts by weight)
[0058] 200 parts of butyl methacrylate, 248 parts of butyl acrylate, 52 parts of methacrylic acid, 5 parts of dibenzoyl peroxide, 500 parts of butyl acetate, 24 parts of sodium hydroxide, and 176 parts of water.
[0059] manufacturing process
[0060] Four-neck flask, stirrer, thermometer, heating device, condenser, reflux device, dropping device.
[0061] 1 Mix 200 parts of butyl methacrylate, 248 parts of butyl acrylate, 52 parts of methacrylic acid, and 3 parts of dibenzoyl peroxide. 24 parts of sodium hydroxide and 176 parts of water were mixed evenly. spare.
[0062] 2 Add 500 parts of butyl acetate into the reaction flask, start stirring, raise the temperature to 125°C, and add the above mixed monomers dropwise.
[0063] Keep warm at 3125°C for 2 hours, and add 1 part of dibenzoyl peroxide.
[0064] Keep warm at 4125°C for 2 hours, and add 1 part of dibenzoyl peroxide.
[0065] Kee...
Embodiment 2
[0067] Synthesis of No. 2 Polyacrylic Resin
[0068] Raw materials (parts by weight)
[0069] 70 parts of butyl methacrylate, 300 parts of butyl acrylate, 130 parts of methacrylic acid, 5 parts of dibenzoyl peroxide, 500 parts of butyl acetate, 60 parts of sodium hydroxide, and 140 parts of water.
[0070] manufacturing process
[0071] Four-neck flask, stirrer, thermometer, heating device, condenser, reflux device, dropping device.
[0072] 1 70 parts of butyl methacrylate, 300 parts of butyl acrylate. 130 parts of methacrylic acid and 3 parts of dibenzoyl peroxide are mixed evenly. 60 parts of sodium hydroxide and 140 parts of water are mixed evenly. spare.
[0073] 2 Add 500 parts of butyl acetate into the reaction flask, start stirring, raise the temperature to 125°C, and add the above mixed monomers dropwise.
[0074] Keep warm at 3125°C for 2 hours, and add 1 part of dibenzoyl peroxide.
[0075] Keep warm at 4125°C for 2 hours, and add 1 part of dibenzoyl peroxide...
Embodiment 3
[0078] Preparation of Copper Chloride Isooctanoate
[0079] 86 parts of copper chloride dihydrate, 72 parts of isooctanoic acid, 20 parts of sodium hydroxide, and 200 parts of water.
[0080] manufacturing process
[0081] Dissolve 20 parts of 1 sodium hydroxide in 100 parts of water. Add 72 parts of isooctanoic acid and stir to react until uniform and transparent.
[0082] 2 Dissolve 86 parts of copper chloride dihydrate in 100 parts of water.
[0083] 3 Add sodium hydroxide-isooctanoic acid-water solution to copper chloride-water solution under stirring, and react. There is precipitation.
[0084] 4 Filter and wash the filter cake with water until the filtrate is colorless (no copper chloride).
[0085] 5 The filter cake was dissolved in 200 parts of butyl acetate solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com