Purifying method of interferon
A purification method and interferon technology, which is applied in the field of interferon purification, can solve the problems of specific activity decline and achieve the effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
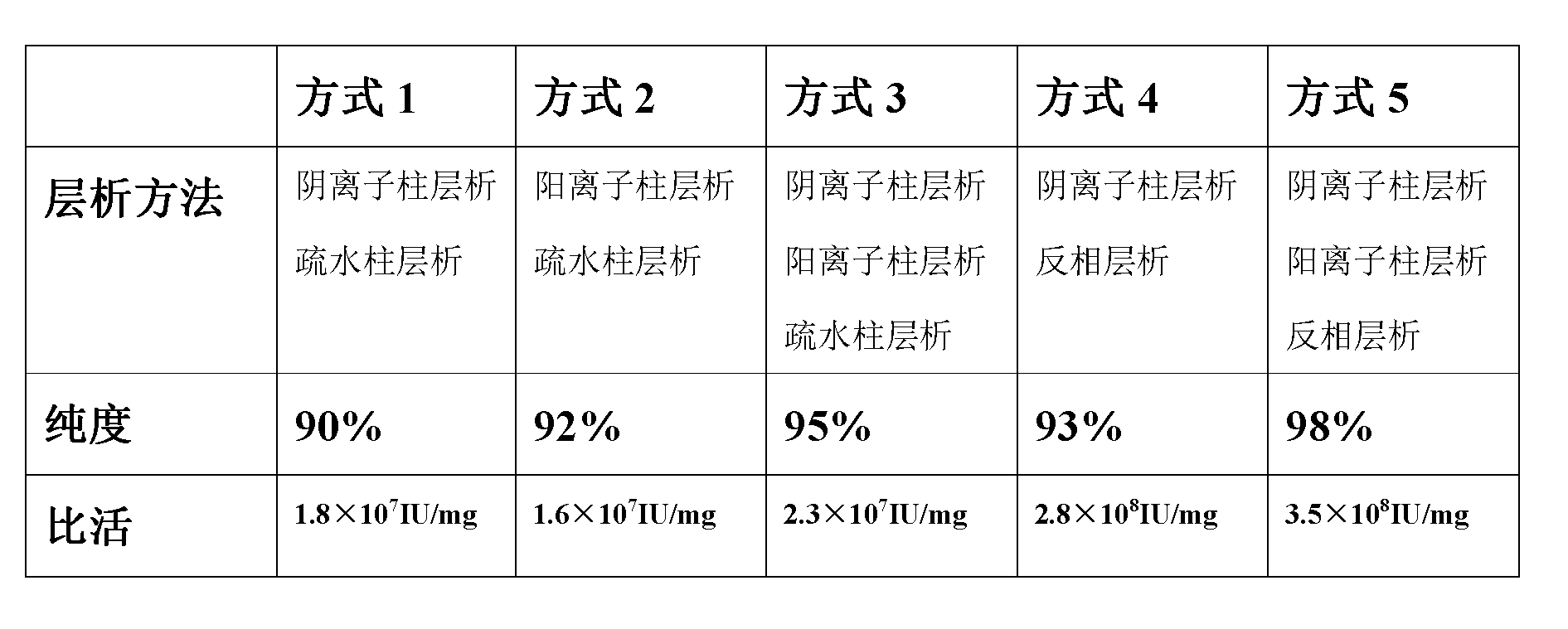
[0034] Anion column chromatography (XK50 / 30 chromatography column, Q sepharose FF filler)
[0035] Use the balance solution (20mmol / L PH8.2 Tris-HCl buffer), adjust the pump flow rate to 30.0-49.9ml / min. Equilibrate to 8-10 times the column bed volume.
[0036] Sample pretreatment: adjust the pH value of the renatured product to 8.2 with 2mol / L pH8.2 Tris-HCl, and then dilute its volume four times with water for injection, which is the loading solution.
[0037] Sample loading: The pump flow rate is 8.0-49.9ml / min when loading the sample. Equilibrate 2-4 column bed volumes with equilibration solution after sample loading.
[0038] Elution: use pre-wash solution (20mmol / L pH7.8 NaH 2 PO 4 -Na 2 HPO 4 Buffer) for pre-washing, adjust the pump flow rate to 30.0-49.9ml / min, and elute 2-4 column bed volumes. Then change the inlet tube to the eluent (20mmol / L pH7.8 NaH2PO4-Na2HPO4 buffer, 0.1MNaCL), start the elution of the target peak, and collect the OD 280 Greater than 0.3...
Embodiment 2
[0045] Cation column chromatography (XK50 / 30 chromatography column, CM Sepharose FF filler)
[0046] Balance: use balance solution (20mmol / L PH4.5 HAc-NaAc buffer), adjust the pump flow rate to 30.0-49.9ml / min, balance 8-10 times the column bed volume.
[0047] Sample pretreatment: adjust the PH value of the renatured product to 4.5 with glacial acetic acid, and then dilute its volume four times with water for injection, which is the sample solution.
[0048] Sample loading: adjust the pump flow rate to 30.0-49.9ml / min, and start sample loading. After loading the sample, switch to the balance solution and balance 2-4 column bed volumes until the recording pen returns to the baseline. Pre-wash with pre-extraction solution (20mmol / L PH4.5HAc-NaAc buffer, 0.15 MNaCL buffer), and adjust the pump flow rate to 30.0-49.9ml / min until the impurity peak is washed down. Change to the eluent (50mmol / L PH4.5HAc-NaAc buffer, 0.2 MNaCL buffer) to elute the target peak, and collect the elut...
Embodiment 3
[0055] Anion column chromatography (XK50 / 30 chromatography column, Q sepharose FF filler)
[0056] Use the balance solution (20mmol / L PH8.2 Tris-HCl buffer), adjust the pump flow rate to 30.0-49.9ml / min. Equilibrate to 8-10 times the column bed volume.
[0057] Sample pretreatment: adjust the pH value of the renatured product to 8.2 with 2mol / L pH8.2 Tris-HCl, and then dilute its volume four times with water for injection, which is the loading solution.
[0058] Sample loading: The pump flow rate is 8.0-49.9ml / min when loading the sample. Equilibrate 2-4 column bed volumes with equilibration solution after sample loading.
[0059] Elution: use pre-wash solution (20mmol / L pH7.8 NaH 2 PO 4 -Na 2 HPO 4 Buffer) for pre-washing, adjust the pump flow rate to 30.0-49.9ml / min, and elute 2-4 column bed volumes. Then change the inlet tube to the eluent (20mmol / L pH7.8 NaH2PO4-Na2HPO4 buffer, 0.1MNaCL), start the elution of the target peak, and collect the OD 280 Greater than 0.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com