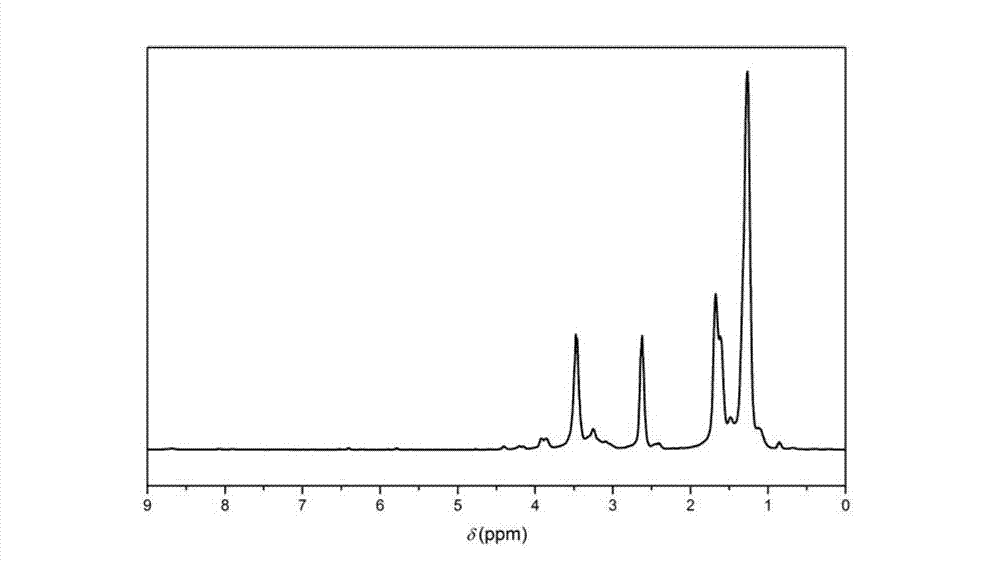
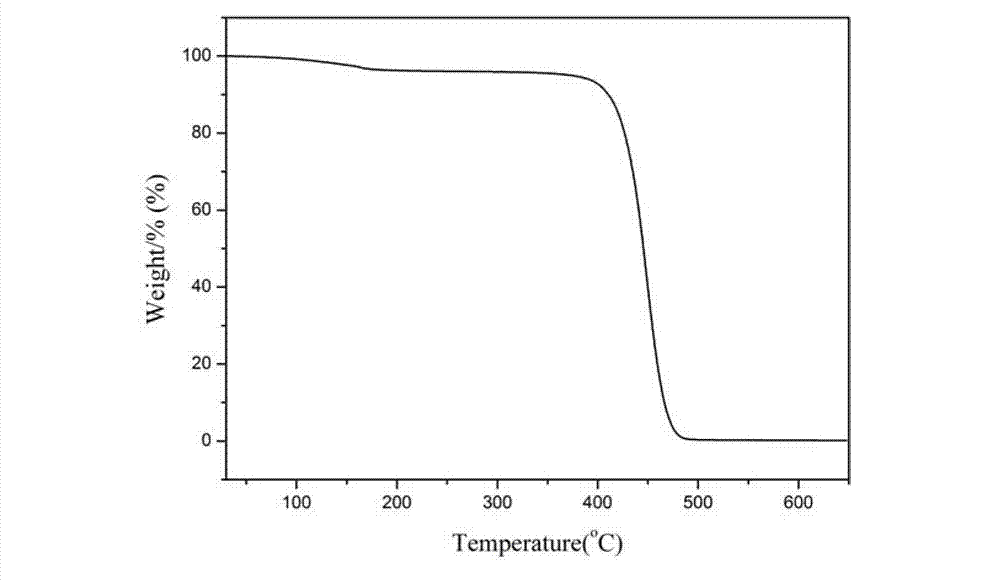
Biologic polyamide quadripolymer and synthesis method thereof
A technology of bio-based polyamide and tetrapolymer, applied in the preparation of bio-based polyamide, aliphatic polyamide tetrapolymer and its preparation field, to achieve low degradation rate, expand the application range, and solve the problem of depletion of petroleum resources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
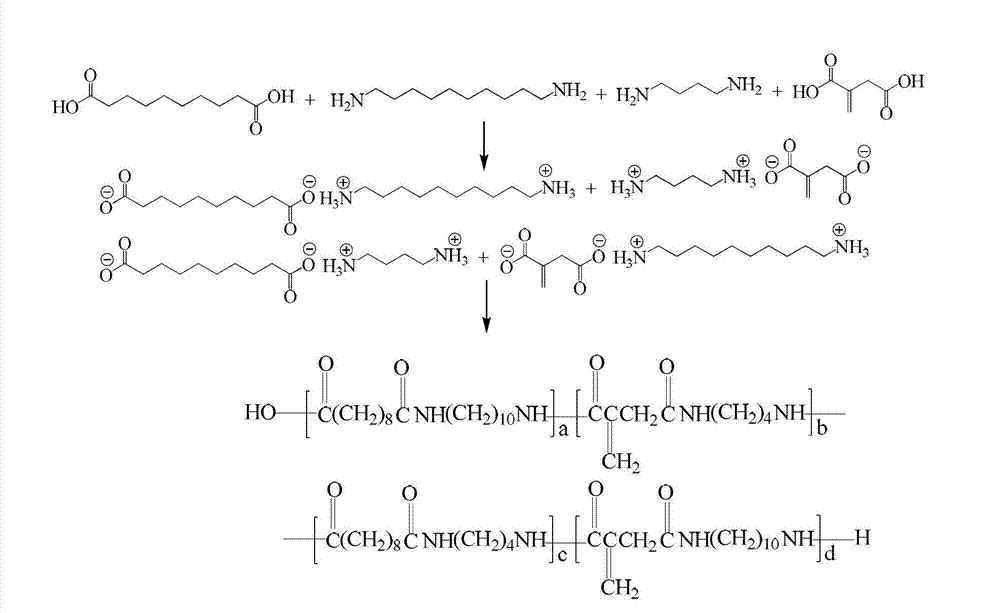
[0031] Add 1.9515g (ie 0.015mol) of itaconic acid and 17.1913g (ie 0.085mol) of sebacic acid into 150ml of absolute ethanol and heat to 65°C to dissolve them completely. Add 1.7630 g (ie 0.02 mol) of butanediamine and 13.9464 g (ie 0.08 mol) of decanediamine into 100 ml of absolute ethanol, and heat to 50° C. to completely dissolve them.
[0032] Pour the ethanol solution of the above-mentioned mixed diamine monomer into the ethanol solution of the above-mentioned mixed diacid monomer, continue stirring at 50°C for 10 minutes, obtain crystals after cooling and crystallization, and suction filtration, wash the obtained crystals with absolute ethanol 3 times, Then transfer it to a petri dish and put it into a vacuum drying oven at 30° C. for 12 hours to obtain the amide salt.
[0033]Add the above-mentioned amide salt, hydroquinone (0.03% of the total mass of amide salt) and phosphorous acid (0.01% of the total mass of amide salt) into a three-necked flask equipped with mechanic...
Embodiment 2
[0035] Add 1.9515g (ie 0.015mol) of itaconic acid and 17.1913g (ie 0.085mol) of sebacic acid into 150ml of absolute ethanol and heat to 65°C to dissolve them completely. Add 2.6445g (ie 0.03mol) of butanediamine and 12.2031g (ie 0.07mol) of decanediamine into 100ml of absolute ethanol, and heat to 50°C to dissolve them completely.
[0036] Pour the ethanol solution of the above-mentioned mixed diamine monomer into the above-mentioned ethanol solution of the mixed diacid monomer, continue stirring at 50°C for 10 minutes, obtain crystals after cooling and crystallization, and suction filtration, wash the obtained crystals with absolute ethanol 3 times, Then transfer it to a petri dish and put it into a vacuum drying oven at 30° C. for 12 hours to obtain the amide salt.
[0037] Add the above-mentioned amide salt and phosphorous acid (0.04% of the total mass of amide salt) into a three-necked flask equipped with mechanical stirring and a thermometer, and vacuum to completely remo...
Embodiment 3
[0039] Add 1.9515g (ie 0.015mol) of itaconic acid and 17.1913g (ie 0.085mol) of sebacic acid into 150ml of absolute ethanol and heat to 65°C to dissolve them completely. Add 3.5260g (ie 0.04mol) of butanediamine and 10.4598g (ie 0.06mol) of decanediamine into 100ml of absolute ethanol, and heat to 50°C to dissolve them completely.
[0040] Pour the ethanol solution of the above-mentioned mixed diamine monomer into the above-mentioned ethanol solution of the mixed diacid monomer, continue stirring at 50°C for 12 minutes, obtain crystals after cooling and crystallization, and suction filtration, and wash the obtained crystals with absolute ethanol for 5 times. Then transfer it to a petri dish and put it into a vacuum drying oven at 30° C. for 12 hours to obtain the amide salt.
[0041] Add the above-mentioned amide salt and hydroquinone (0.01% of the total mass of the amide salt) into a three-necked flask equipped with mechanical stirring and a thermometer, and vacuumize to comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com