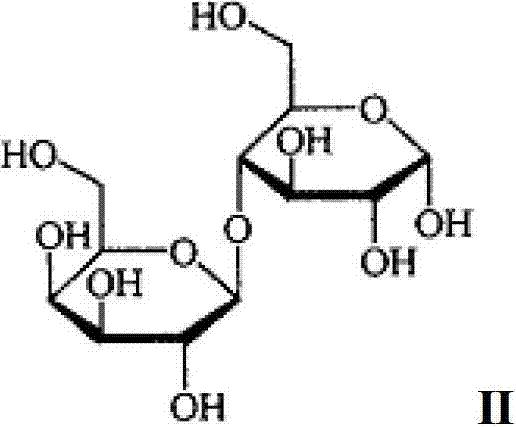
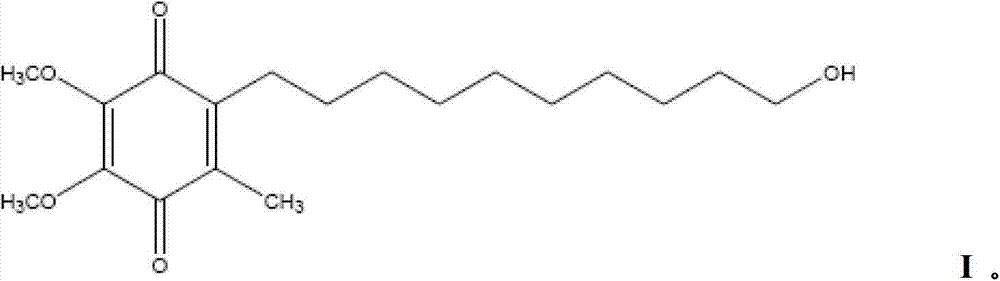
Oral solid medicine composition for nervous system diseases
A technology of adhesives and tablets, which can be used in nervous system diseases, drug combinations, cardiovascular system diseases, etc., can solve the problems of left ventricular enlargement and reduction, achieve elimination half-life, low toxic and side effects, and improve brain energy metabolism. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: prepare pharmaceutical composition (tablet) of the present invention
[0083] Plain Tablet Prescription:
[0084]
[0085] Preparation method:
[0086] i) Fully mix the idebenone, anhydrous lactose, and half of the disintegrant;
[0087] ii) The binder povidone K30 is made into an aqueous solution (8%), and the powder material obtained in the previous step is processed into a soft material, granulated, and dried;
[0088] iii) Mix the dried granules with the rest of the disintegrant and lubricant and / or glidant, and press it into the present invention
[0089] Tablets, which may be called tablet cores or plain tablets;
[0090] iv) Coating the tablet obtained in the above steps (Using Opadry, it is formulated into a 14% aqueous suspension immediately before use, and the coating weight increases by about 3%) to obtain a coated tablet of the pharmaceutical composition of the present invention.
Embodiment 2
[0091] Embodiment 2: prepare pharmaceutical composition (tablet) of the present invention
[0092] Plain Tablet Prescription:
[0093]
[0094] Preparation method: basically with embodiment 1. It has been determined that the weight ratio of idebenone to anhydrous lactose in the tablet is 30:80.23.
Embodiment 3
[0095] Embodiment 3: prepare pharmaceutical composition (tablet) of the present invention
[0096] Plain Tablet Prescription:
[0097]
[0098] Preparation method: basically with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com