Synthesis method of delta-dodecalactone
A synthesis method and technology of laurolactone, applied in the field of synthesis of delta-dodecolactone, can solve the problems of difficult industrialization, low yield, long steps, etc., and achieve reduction of cardiovascular disease, energy consumption, operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
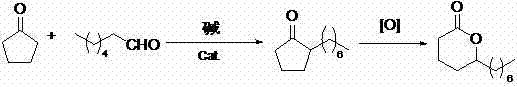
[0020] (1) Synthesis of 2-heptylcyclopentanone
[0021] Add 1780g of 1.20% sodium hydroxide solution and 50.0g of Pd / C (50% water) catalyst into a 5L reactor, N 2 Replace three times, raise the temperature to 90 ℃, pass hydrogen and keep the pressure at 3.5MPa, add dropwise a mixture of 861.4g (10.24mol) cyclopentanone and 1216.9g (10.66mol) n-heptanal, dropwise add in 4 hours, continue to keep warm after dropwise addition Pressure holding reaction 0.5h, release pressure, N 2 Replaced three times, filtered after cooling down to 60°C, recovered the catalyst and used mechanically, separated, and distilled the oil layer to recover the crude product after cyclopentanone was rectified to obtain 1570 g of 2-heptylcyclopentanone, with a content of 95% by GC analysis.
[0022] (2) Synthesis of δ-Lauryl Lactone
[0023] Add 1500g of toluene to the three-necked flask as a solvent, then add 1570g of crude 2-heptylcyclopentanone, and slowly add 3734g of 20% peracetic acid dropwise under...
Embodiment 2
[0025] (1) Synthesis of 2-heptylcyclopentanone
[0026] Add 2897g of 5.0% potassium hydroxide solution and 70.0g of Pd / C (50% water) catalyst into a 5L reactor, N 2 Substitute three times, raise the temperature to 50°C, pass hydrogen and keep the pressure at 2.0MPa, add dropwise a mixture of 861.4g (10.24mol) cyclopentanone and 586.9g (5.14mol) n-heptanal, dropwise add in 2 hours, and continue to keep warm after the dropwise addition Hold the pressure for 15 minutes, release the pressure, N 2 Replaced three times, filtered after cooling down to 60°C, recovered the catalyst and used it mechanically, separated, and distilled the oil layer to recover the crude product after cyclopentanone was rectified to obtain 675 g of 2-heptylcyclopentanone with a content of 95% by GC analysis.
[0027] (2) Synthesis of δ-Lauryl Lactone
[0028] Add 1350.0g of toluene to the three-necked flask as a solvent, then add 675g of crude 2-heptylcyclopentanone, and slowly add 272.6g of 50.8% hydroge...
Embodiment 3
[0030] (1) Synthesis of 2-heptylcyclopentanone
[0031] Add 1600g of 0.5% lithium hydroxide solution and 3.2g of Pd / C (50% water) catalyst into a 5L reactor, N 2 Substitute three times, raise the temperature to 100°C, pass hydrogen and keep the pressure at 5.0MPa, add dropwise a mixture of 861.4g (10.24mol) cyclopentanone and 2338.4g (20.48mol) n-heptanal, dropwise add in 5 hours, and continue to keep warm after the dropwise addition Reaction under pressure for 3h, release pressure, N 2 Replaced three times, filtered after cooling down to 60°C, recovered the catalyst and used mechanically, separated, and distilled the oil layer to recover the crude product after cyclopentanone was rectified to obtain 1413g of 2-heptylcyclopentanone, the content of GC analysis was 95.5%.
[0032] (2) Synthesis of δ-Lauryl Lactone
[0033] Add 70.7g of xylene to the three-necked flask as a solvent, then add 1413g of crude 2-heptylcyclopentanone, and slowly add 4420g of 20% peracetic acid dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com