Immunogenic composition
A technology of immunogenicity and composition, which is applied in the field of immunogenic composition, can solve the problems of inconsistency in performance, slow-release performance, inference of adjuvant function, and failure to realize it, and achieve a powerful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Synthesis of dextran-poly(lactic acid-glycolic acid) (PLGA)
[0105] (1-1) Synthesis of TMS-dextran (compound (1))
[0106] Add dextran (Nacalai Tesque Co., Ltd., Nacalai standard super qualified product, number average molecular weight: 13,000, 5.0 g) into formamide (100 ml), and heat to 80°C. To this solution was added dropwise 1,1,1,3,3,3-hexamethyldisilazane (100 ml) over 20 minutes. After completion of the dropwise addition, the mixture was stirred at 80° C. for 2 hours. After completion of the reaction, the reaction solution was returned to room temperature, and the two layers were separated with a separatory funnel. After concentrating the upper layer under reduced pressure, methanol (300 ml) was added, and the obtained solid was filtered and dried to obtain TMS-dextran (compound (1)) (11.4 g) as a white solid.
[0107] (1-2) Synthesis of dextran-PLGA (compound (2))
[0108] Compound (1) (0.5 g) and potassium tert-butoxide (35 mg) were heated and dr...
Embodiment 2
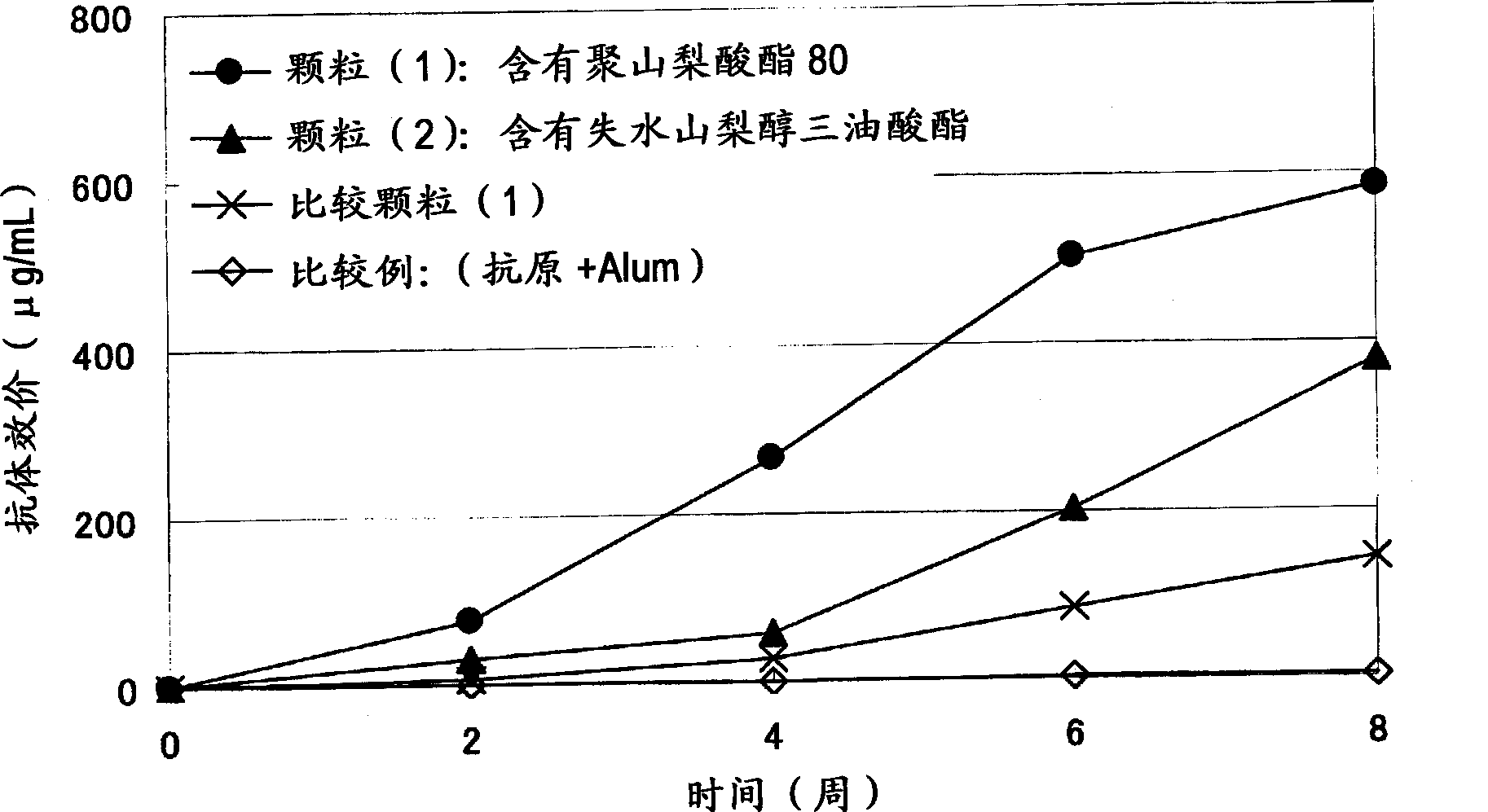
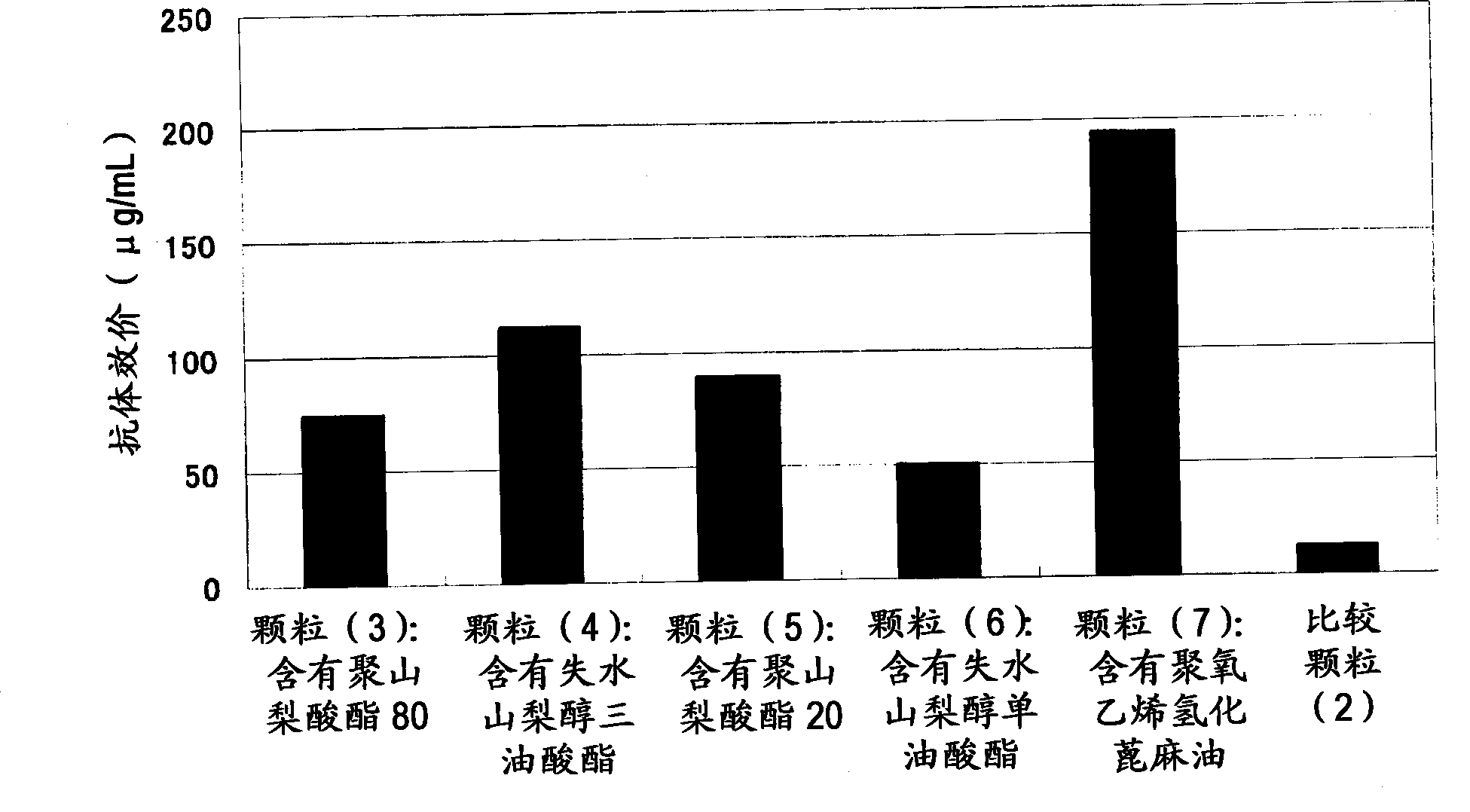
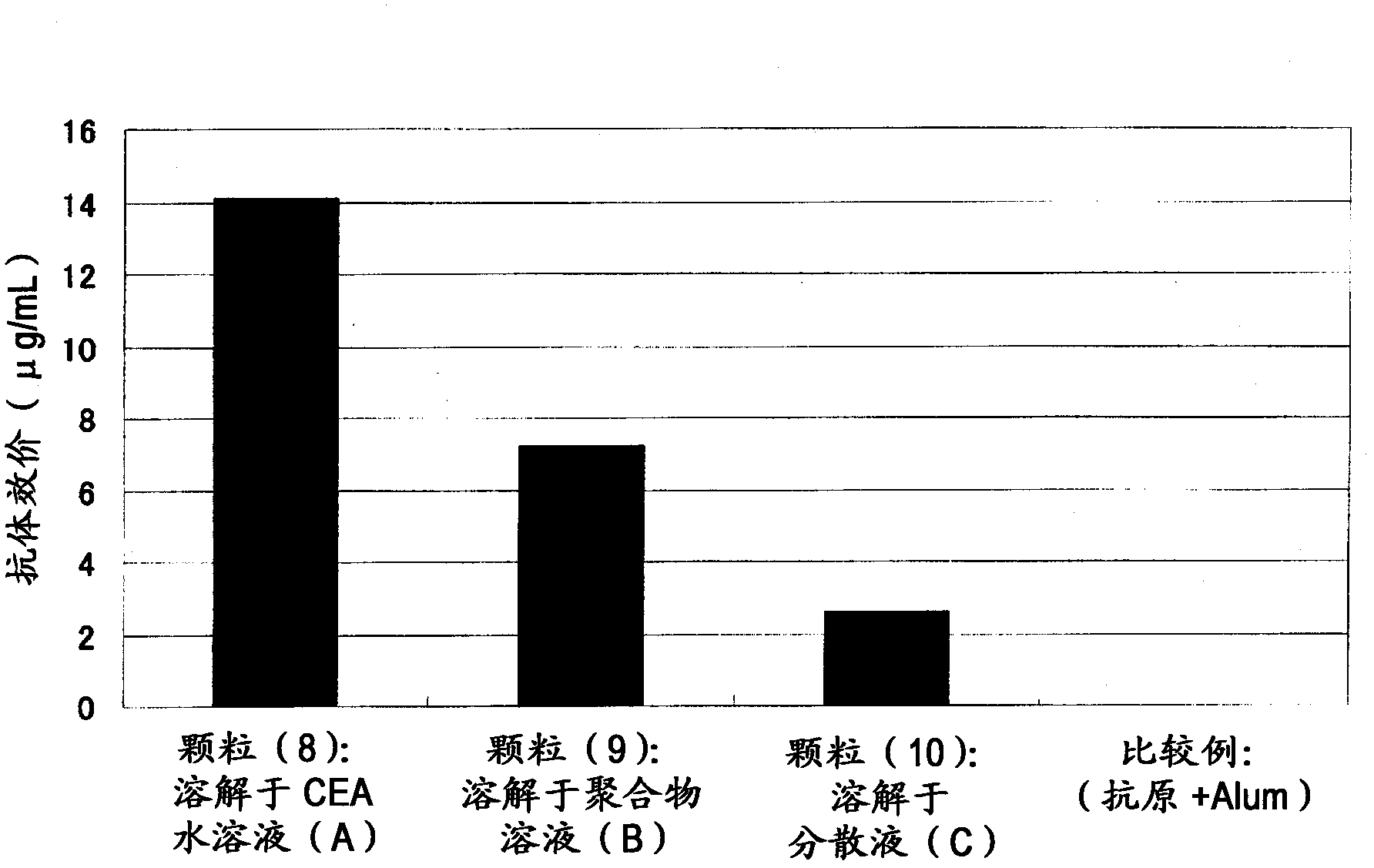
[0109] Example 2 Preparation of Immunogenic Microparticles (Particles (1)-(10) and Comparative Particles (1), (2))
[0110] 5 mg of dextran-poly(lactic-glycolic acid) (PLGA) (compound (2)) of Example 1 was dissolved in 100 μl of dimethyl carbonate to prepare a 50 mg / ml amphiphilic polymer solution (B). After adding 20 μl of tert-butanol to the amphiphilic polymer solution (B), 50 μl of 0.025% (w / v) CEA (carcinoembryonic antigen) (COSMO BIO)) aqueous solution (A) was added dropwise, and stirred with a VORTEX mixer Make an inverse emulsion.
[0111] The inverse emulsion was pre-frozen with liquid nitrogen, and then freeze-dried for 24 hours with a freeze dryer (EYELA, FREEZEDRYERFD-1000) at a trap cooling temperature of -45°C and a vacuum of 20 Pa. The obtained solid content was dispersed in 200 µl of dimethyl carbonate to prepare a dispersion (C). Add the dispersion (C) dropwise to 2 ml of an aqueous solution containing 10% (w / v) of the surface modifier (polyvinyl alcohol or ...
Embodiment 3
[0117] Example 3 Determination of Antigen Embedding Rate in Immunogenic Microparticles
[0118]
[0119] The immunogenic microparticles (particles (3) to (7) and comparative particle (2)) produced by the method of Example 2 were suspended in 200 μl of phosphate-buffered saline to prepare a particle suspension. 100 µl of the particle suspension was transferred to an Eppendorf tube, 1 ml of distilled water was added, and the particles were precipitated by centrifugation at 13,000 rpm for 10 minutes. After removing the supernatant, the particles were resuspended in 1 ml of distilled water, and the particles were re-sedimented by centrifugation under the above conditions. After removing the supernatant, the particles were dissolved in 0.5 ml of a mixed solution obtained by mixing dichloromethane and acetone at a ratio of 1:3. The particle solution was centrifuged at 13,000 rpm for 10 minutes to precipitate CEA. After removing the supernatant, the precipitate was dissolved in 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com