Venlafaxine sustained-release capsule and preparation process thereof
A technology for sustained-release capsules and venlafaxine, which is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, organic active ingredients, etc., can solve the problem of accelerating drug release, increasing osmotic pressure, and increasing production costs. and other problems, to achieve the effect of slowing the release rate, reducing the solubility, and reducing the osmotic pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] A preparation method for venlafaxine sustained-release capsules, comprising the following steps:
[0047] 1) Weigh the adhesive according to the prescription amount, and prepare a 10-30% (g / mL) adhesive solution with ethanol for later use;
[0048] 2) Put the blank core in the granulator, weigh the binder solution and filler according to the prescription, spray the liquid and powder, so that the binder and filler are evenly dispersed and deposited on the blank core to form an isolated pellet. Floor;
[0049] 3) take binder solution and venlafaxine by prescription quantity, spray liquid and apply powder, make binder and venlafaxine evenly disperse and deposit on the isolation layer, make drug-containing layer, obtain drug-containing micropill;
[0050] 4) Then, weigh the binder solution and the filler according to the prescription amount, spray the liquid and apply the powder, so that the binder and the filler are evenly dispersed and deposited on the drug-containing la...
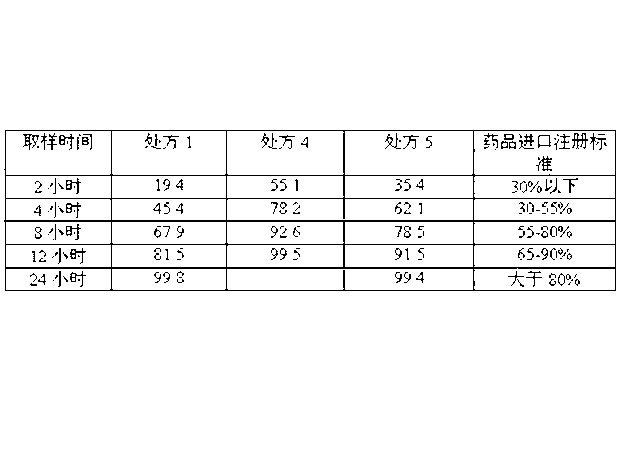
Embodiment 1
[0057] The sustained-release pellets consist of the following components by weight from the inside to the outside:
[0058] Sucrose blank core 10
[0059] isolation layer 5
[0060] Drug-containing layer 20
[0061] Slow Release Protective Layer 10
[0062] 1) Weigh ethyl cellulose and enteric acrylic resin in a weight ratio of 1:0.5 and mix them evenly as a binder, prepare a 15% (g / mL) binder solution with ethanol for later use; weigh talcum powder as a filler agent spare;
[0063] 2) Put the blank pellet core in the centrifugal granulator, take the binder solution and filler (the weight ratio of binder to filler is 1:4), spray liquid and powder, make the binder and filler Evenly disperse and deposit on the blank pellet core to make an isolation layer;
[0064] 3) Next, take the binder solution and venlafaxine (the weight ratio of binder to venlafaxine is 1:7), spray liquid and powder, so that the binder and venlafaxine are uniformly dispersed and deposited on the isolatio...
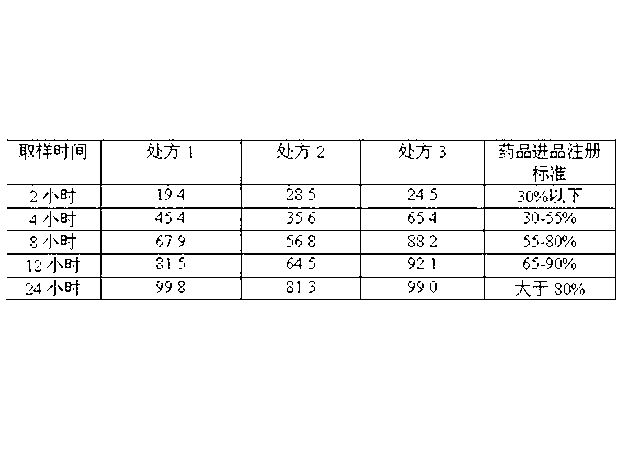
Embodiment 2
[0069] The sustained-release pellets consist of the following components by weight from the inside to the outside:
[0070] Starch blank core 15
[0071] isolation layer 10
[0072] Drug-containing layer 10
[0073] Slow Release Protective Layer 20
[0074] 1) Weigh ethyl cellulose and enteric acrylic resin in a weight ratio of 1:0.2 and mix them evenly as a binder, prepare a 10% (g / mL) binder solution with ethanol for later use; weigh talcum powder as a filler agent spare;
[0075] 2) Put the blank pellet core in the centrifugal granulator, take the binder solution and filler (the weight ratio of binder to filler is 1:6), spray liquid and powder, make the binder and filler Evenly disperse and deposit on the blank pellet core to make an isolation layer;
[0076] 3) Next, take the binder solution and venlafaxine (the weight ratio of binder to venlafaxine is 1:5), spray liquid and powder, so that the binder and venlafaxine are uniformly dispersed and deposited on the isolat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com