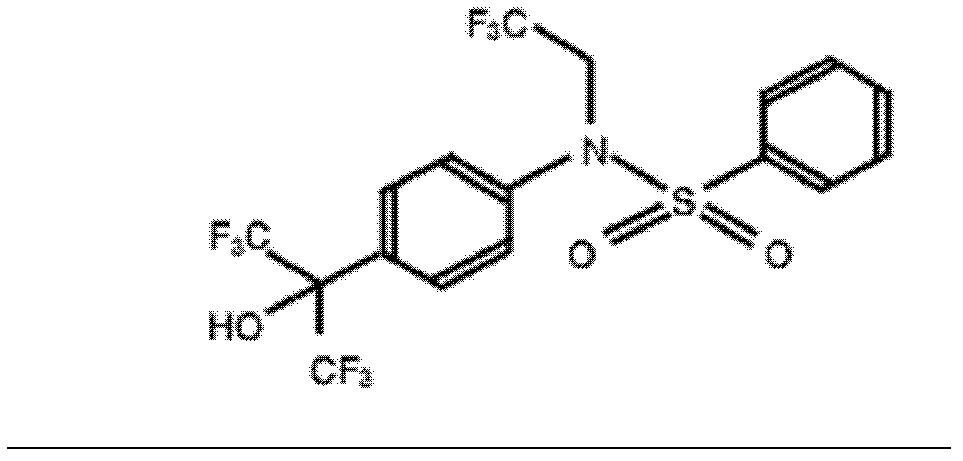
Application of T0901317 in preparation of drugs treating or preventing hepatitis C
A hepatitis C virus and drug technology, which is applied in the field of medicine, can solve the problem that there is no application of T0901317 in anti-hepatitis C virus, and achieve the effect of improving the cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
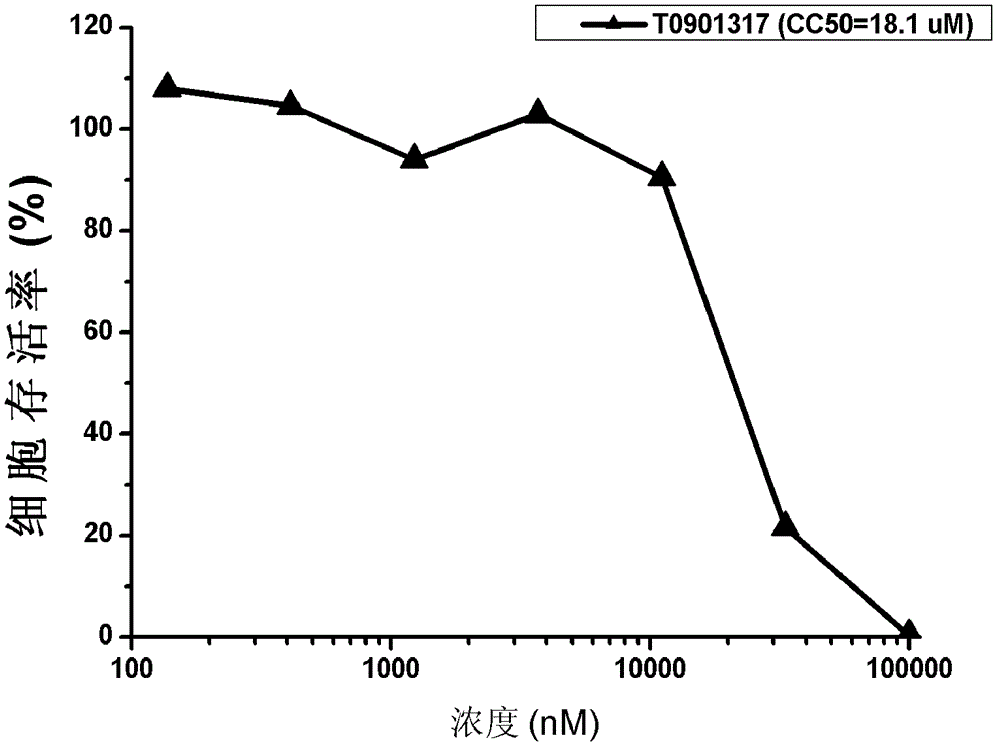
[0035] Example 1: Evaluation of T0901317 Anti-Hepatitis C Virus Activity
[0036] 1. Experimental materials
[0037] 1.1 Cells, plasmids, viruses and drugs
[0038] Huh7.5.1 cells were donated by Dr.F.V.Chisari; plasmid pJFH1 containing the complete genome sequence of HCV type 2a JFH1 strain was donated by Prof. Dr. Takaji Wakita; plasmid pEGFP-N1 was purchased from Clontech; plasmid pGL4.70[hRLuc] was purchased from promega company. The virus JFH1-Luc-5AGFP with double reporter genes was prepared by our laboratory; T0901317 was purchased from sigma company.
[0039] 1.2 Reagents
[0040] DMEM medium was purchased from GIBCO Company; Renilla luciferase detection kit was purchased from Promega Company; anti-HCV NS3 mouse monoclonal antibody was purchased from Henan Bioengineering Technology Research Center; anti-GAPDH mouse monoclonal antibody was purchased from Beijing Zhongshan Golden Bridge Company; HRP-labeled goat anti-mouse secondary antibody was purchased from Thermo...
Embodiment 2
[0058] Embodiment 2: The research of T0901317 enters the influence of hepatitis C pseudovirus (HCVpp)
[0059] 1. Experimental materials
[0060] 1.1 Cells, viruses and drugs
[0061] Huh7.5.1 cells; T0901317; plasmid PNL4.3-R-E-Luc and plasmid pVpack-VSV-G containing the VSV viral envelope protein were kindly provided by the Division of AIDS Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The plasmids containing HCV1a and 1b envelope proteins were donated by Mr. Qi Zhongtian.
[0062] 1.2 Reagents
[0063] DMEM medium was purchased from GIBCO; transfection reagent Lipofectamine2000 was purchased from Invitrogen; Firefly luciferase detection kit was purchased from Promega.
[0064] 1.3 Experimental Instruments
[0065] The detector is a product of Promega Company.
[0066] 2. Experimental methods and results
[0067] 2.1 Compared with the live virus, the HCV pseudovirus has similar cell infection characteristics, which ca...
Embodiment 3
[0069] Example 3: Combination study of T0901317 and other drugs with anti-HCV activity.
[0070] 1. Experimental materials
[0071] 1.1 Cells, viruses and drugs
[0072] Huh7.5.1; virus JFH1-Luc-5AGFP; T0901317; CsA (purchased from Sigma Company) and MK-7009 (purchased from Zannan Company).
[0073] 1.2 Reagents
[0074] DMEM medium was purchased from GIBCO; Renilla luciferase detection kit was purchased from Promega.
[0075] 1.3 Experimental Instruments
[0076] The detector is a product of Promega Company.
[0077] 2. Experimental methods and results
[0078] 2.1 Divide Huh7.5.1 cells into 8×10 cells 3 Each cell / well was inoculated in a 96-well cell culture plate, cultured in a 37°C cell culture incubator for 14-18 hours, and then used after the cells grew into a monolayer. Cyclosporine A (CsA) or MK-70092-fold gradient dilution was added to the orifice plate as a control group for separate medication, and each group had 3 repetitions; in addition, 500 nM T0901317 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com