Application of cepharanthine in preparation of anti-influenza virus drugs
An anti-influenza virus, fenugreek technology, applied in antiviral agents, pharmaceutical formulations, plant raw materials, etc., can solve the problem that the application of fenugreek in anti-influenza virus has not been reported, and achieve broad-spectrum antiviral activity, good Application prospects, the effect of suppressing copying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
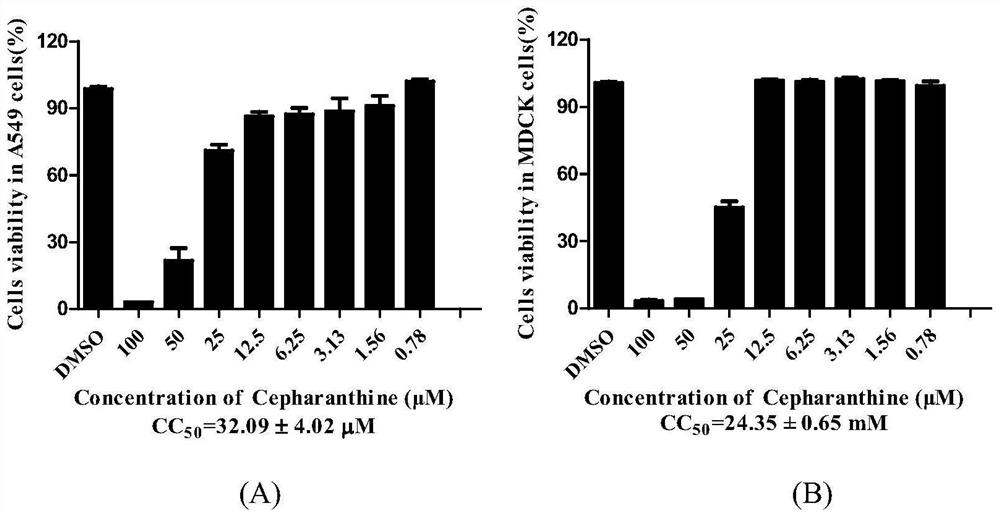
[0045] Example 1: Detection of Stephalin on Cytotoxicity
[0046] The cytotoxicity of cephaglidin is detected by MTT method, so as to determine the concentration of the drug. The specific method is as follows:
[0047] MDCK or A549 cells by 1×10 4 / well seeded in 96-well plate at 37°C, 5% CO 2 Cultured in a constant temperature cell culture incubator until monolayer, stepwise dilute stepherin with serum-free DMEM or 1640 (DMEM medium for MDCK cells, 1640 medium for A549 cells) and add it to a 96-well plate, 200 μL per well , continue to cultivate for 48h. Discard the culture supernatant, add 100 μL of 1640 or DMEM medium containing 0.5 mg / mL MTT to each well, and incubate at 37 °C for 4 h. DMSO was used as the control group. A multifunctional microplate reader (Genios Pro, Tecan, US) was used to detect the absorbance at 570nm, and the cell survival rate was used as an indicator of the toxicity of stephalin to MDCK or A549 cells.
[0048] Cell viability (%)=E / N×100
[004...
Embodiment 2
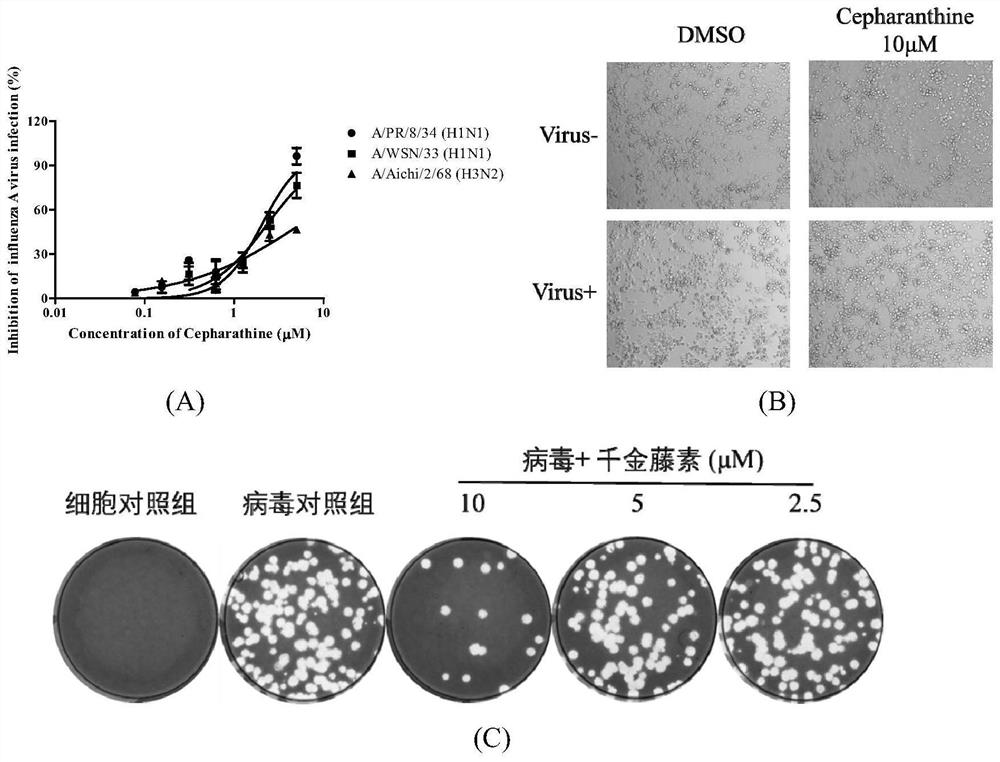
[0052] Example 2: Anti-Influenza A Virus Activity Detection of Stephalin in Vitro
[0053] In the in vitro antiviral experiment of the present invention, various subtypes of influenza A viruses are involved, including H1N1 and H3N2, and the specific methods are as follows:
[0054] MDCK cells by 2 x 10 4 / well seeded in 96-well plate at 37°C, 5% CO 2 cultured to a monolayer in a constant temperature cell incubator. Use 100TCID 50 H3N2, PR8 or WSN-infected cells, 100 μL per well, incubated at 37°C for 1 h, discarded the virus solution, and added stepped stepherin diluted in DMEM (containing 1 μg / mL TPCK), 200 μL per well, continued to culture for 48 h, combined with MTT method and plaque assay to detect the antiviral activity of stepherin. Calculate the half effective concentration IC by observing the cytopathic phenomenon (CPE) caused by the virus and detecting the survival rate of the cells 50 .
[0055] Virus inhibition rate (%)=1-(E / N) / (P-N)×100
[0056] E is the abs...
Embodiment 3
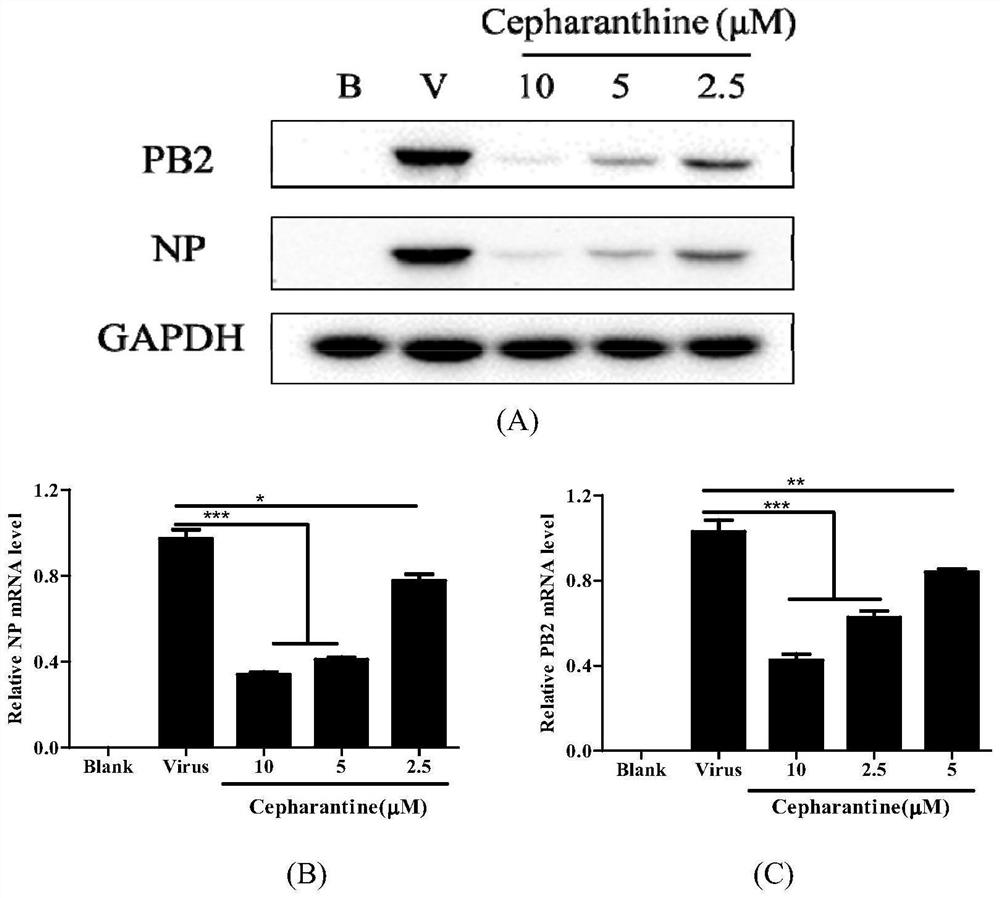
[0061] Example 3: Inhibitory Experiment of Stephalin on the Replication of Influenza A Virus
[0062] In order to evaluate the inhibitory effect of stepherin on the replication of influenza virus, the present invention uses methods such as q-PCR and Western blotting to detect the influence of stepherin on virus replication from the expression levels of genes and proteins respectively. The specific methods are as follows:
[0063] q-PCR and Western blot: A549 cells by 4×10 5 / well seeded in a 6-well plate at 37 °C, 5% CO 2 cultured to a monolayer in a constant temperature cell incubator. Infect the cells with 100TCID50 of WSN, 1mL per well, incubate at 37°C for 1h, discard the virus solution, add Stephalogenin gradiently diluted in DMEM (containing 1μg / mL TPCK), 1mL per well, continue to culture for 24h, then use RNA extraction reagent Kit (provided by Fuji Bio) to fully lyse cells and extract total RNA, samples for q-PCR; or use 75 μL RIPA lysate containing protease and phos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com