Oligopeptide as well as derivatives and application thereof
A technology of derivatives and oligopeptides, applied in the field of oligopeptides and their derivatives and applications, can solve the problems of difficult synthesis and large molecular weight, and achieve the effects of small molecular weight, easy synthesis, and inhibition of influenza virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Virus virulence assay (TCID 50 )
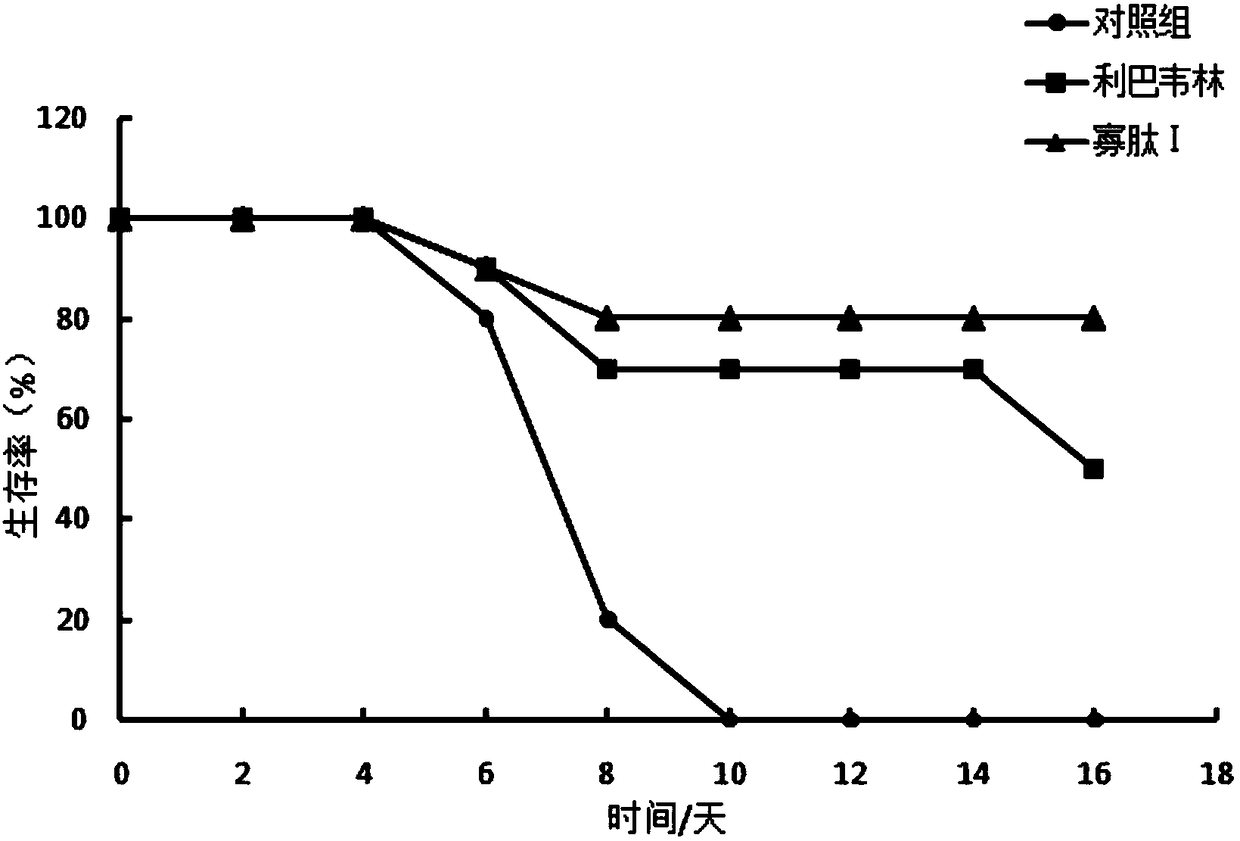
[0036] Influenza H1N1 (FM1), H3N2 (N3), H5N1 and H7N9 virus antigens used in the present invention are virus inactivated antigens with hemagglutination activity, all can cause disease, H1N1, H3N2 are human influenza, H5N1 and H7N9 It is bird flu. Taking H7N9 as an example, the virus virulence was tested to prove its activity. The specific experimental steps and results are as follows:
[0037] After the MDCK cells (influenza virus susceptible canine kidney passage cell strain) in the logarithmic growth phase are laid on a single layer, the influenza virus H1N1 is continuously diluted by 10 times, and 10 -2 ~10 -10 The diluted virus was inoculated into MDCK cells, adsorbed at 37°C for 1 hour, washed twice with PBS, and then replaced with maintenance medium to continue culturing. Normal cells were used as controls, and each well had 4 replicate wells. Observe the cell lesion under an inverted microscope every day, record the lesion de...
Embodiment 2
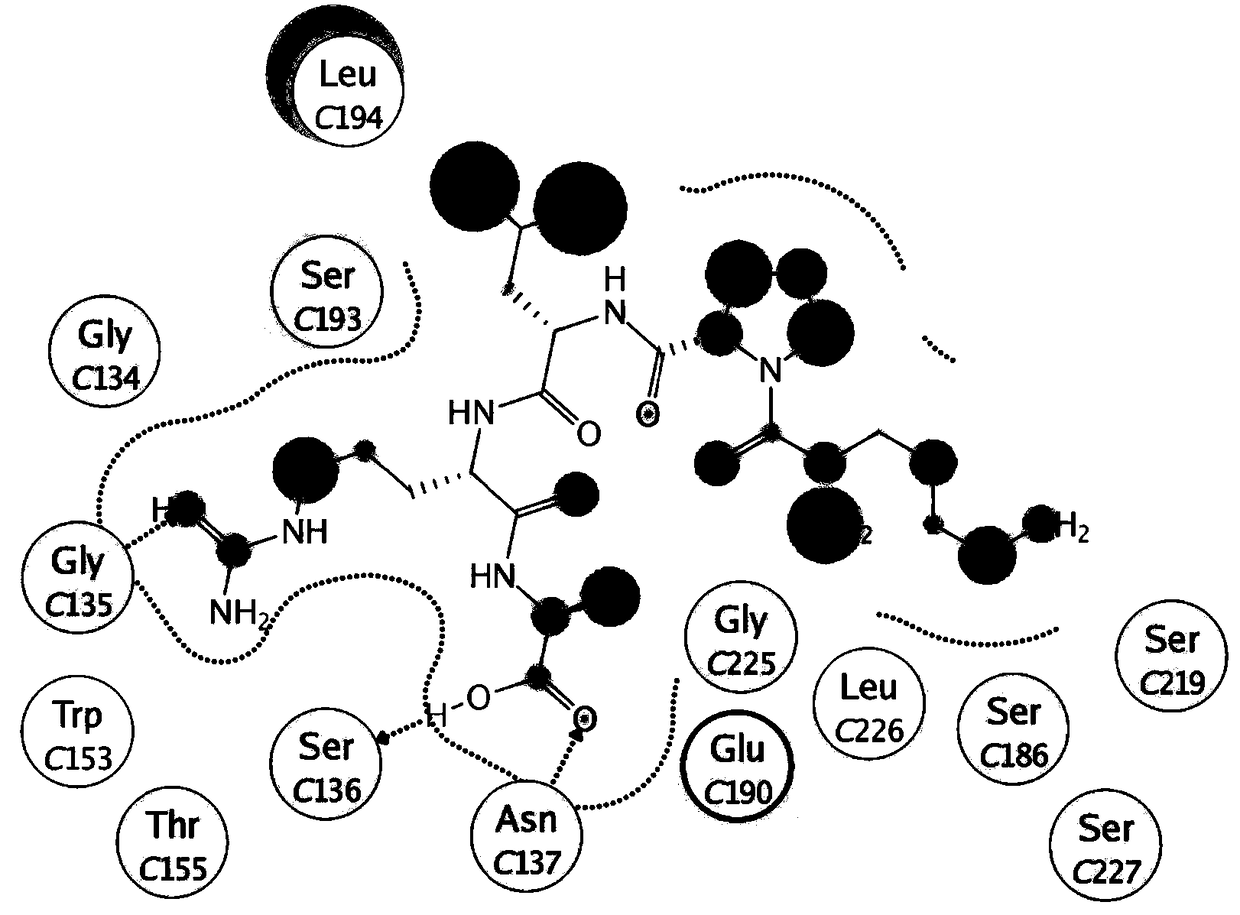
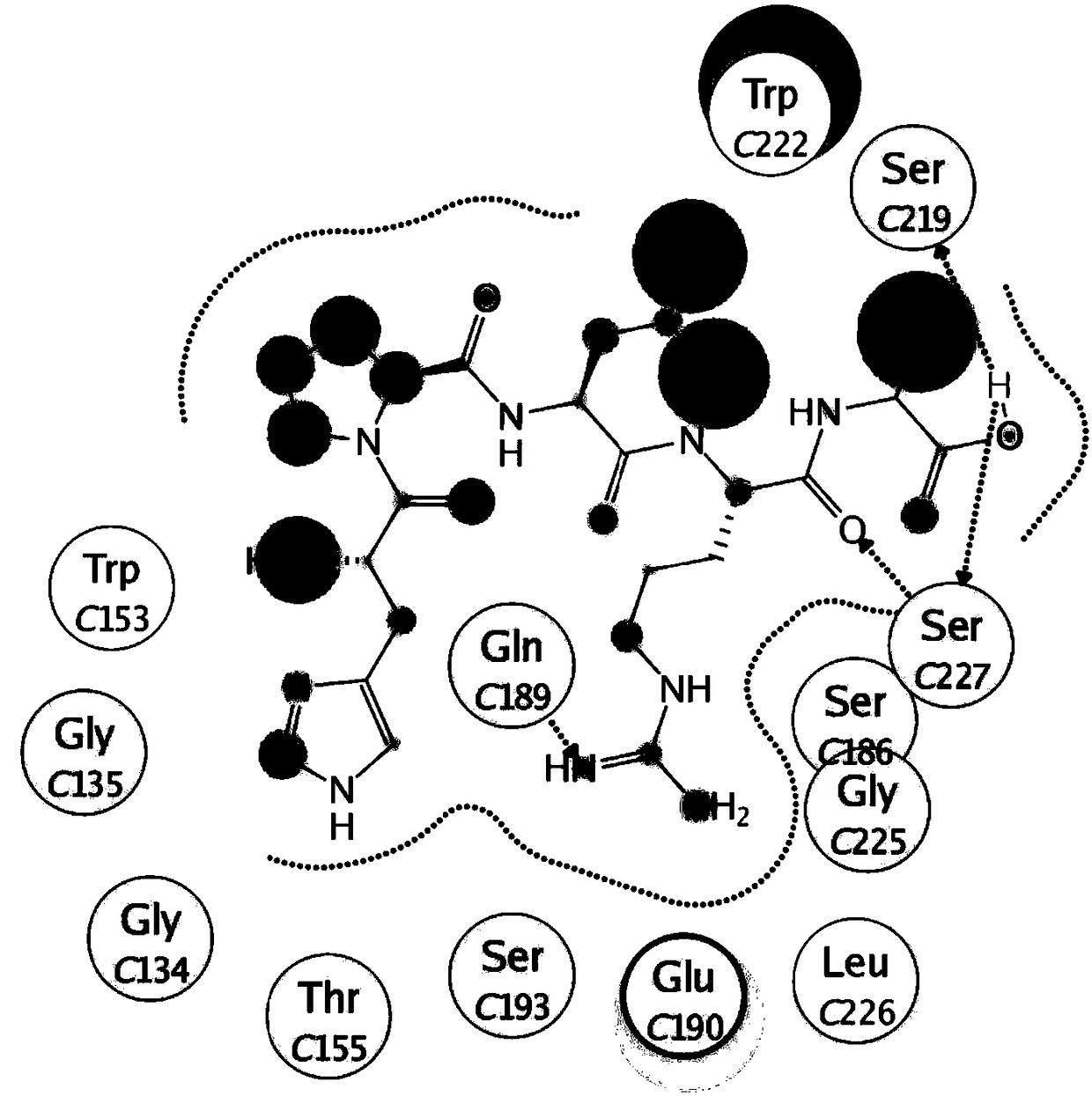
[0046] The present invention uses the protein database (Protein Data Bank) hemagglutinin protein (4BSE) crystal structure as the basis of research, screens out a series of oligopeptides with antiviral activity, its sequence contains X-Y-Leu-Arg-Ala domain, A class of oligopeptide derivatives is obtained by modifying the short peptide X-Y-Leu-Arg-Ala with stearic acid. The general structural formula of this kind of oligopeptide derivatives is C 17 h 35CO-X-Y-Leu-Arg-Ala, wherein X is a positively charged amino acid (such as Lys, His, Arg, etc.); Y is a hydrophobic or positively charged amino acid (such as Pro, Lys, Leu, etc.). These oligopeptide derivatives all have antiviral activity and are expected to be developed into ideal antiviral drugs. The specific structure of oligopeptide derivatives can be:
[0047] Oligopeptide I, C 17 h 35 CO-Lys-Pro-Leu-Arg-Ala; or
[0048] Oligopeptide II, C 17 h 35 CO-His-Pro-Leu-Arg-Ala; or
[0049] Oligopeptide III, C 17 h 35 CO-Ar...
Embodiment 3
[0057] Anti-influenza virus efficacy test method:
[0058] Take cells in good logarithmic growth phase, add 0.25% trypsin digestion solution, blow evenly after digestion and fall off, count, and press 2×10 5 cells / well, seeded in 96-well cell culture plate, placed in constant temperature CO 2 Cultivate in the incubator for 16-24h. Set up normal cell control group, virus infection group, positive drug control group and test drug group respectively. Discard the culture medium, wash three times with PBS, add 100TCID to each well except the normal control group 50 Infectious amount of influenza virus, placed in constant temperature CO 2 The incubator was left to rest for 1 hour, unadsorbed virus was discarded, washed twice with PBS, and then drug-containing maintenance solutions with different concentrations were added. There were 6 doses in the test drug group, each with 3 replicate wells. Then continue to cultivate the cell culture plate with the above-mentioned scheme at 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com