Spirocyclosporine x and its application in the preparation of medicaments for the treatment of influenza A
A type of influenza virus and drug technology, applied in the field of medicine, can solve the problems of increased drug resistance, drug side effects, and no scientific and comprehensive elaboration of antiviral mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
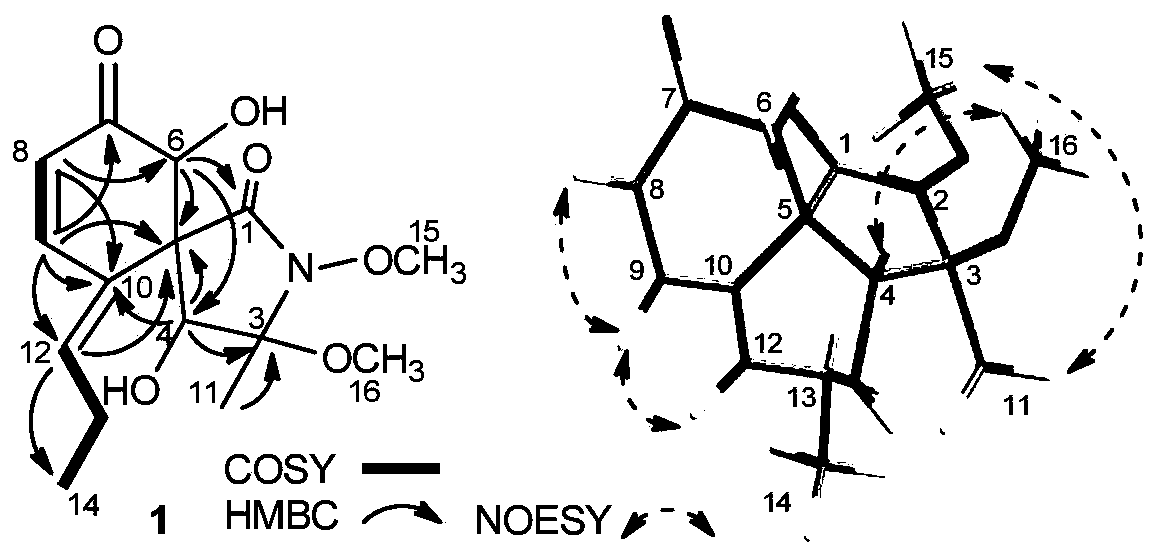
[0036] Example 1 Separation, Preparation and Structure Identification of Compound SSX
[0037]The fungus Cochliobolus lunatus SCSIO41401 is a symbiotic fungus of seaweed, collected from the waters near Yongxing Island in the South China Sea (Journal of Natural Products, Cytotoxic and Antibacterial Eremophilane Sesquiterpenes from the Marine-Derived Fungus Cochliobolus lunatus SCSIO41401, WEI Fang et al., 2018, 81-41405). The strain can be provided by the School of Pharmacy, Southern Medical University. The strain is stored in MB medium, which consists of: agar 15g, malt extract powder 15g, coarse sea salt 24.4g, water 1000mL, pH 7.2-7.4.
[0038] Take a small amount of slant bacteria for seed fermentation culture (medium: 6.25g maltose, 6.25g malt extract powder, 1g yeast extract powder, 6.25g peptone, 1.25g potassium dihydrogen phosphate, 20g sea salt, 1000mL distilled water, pH 7.0), 500 100 ml in an Erlenmeyer flask, culture conditions: 25°C, 180 rpm. After 3 days of cultu...
Embodiment 2
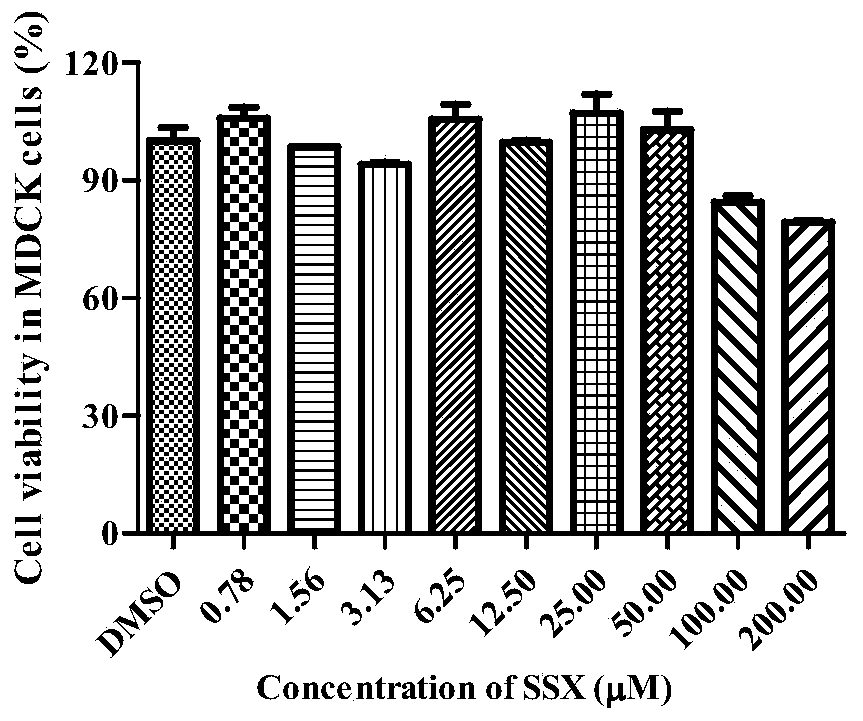
[0046] The cytotoxicity detection of embodiment 2 compound SSX
[0047] The cytotoxicity of compound SSX was detected by MTT method. The specific method is as follows:
[0048] MDCK cells by 1 x 10 4 / well seeded in 96-well plate at 37°C, 5% CO 2 Cultured in a constant temperature cell culture incubator until monolayer, the compound SSX diluted serially was added to a 96-well plate, 200 μl per well, and cultured for 48 hours. The culture supernatant was discarded, and 100 μL of DMEM medium containing 0.5 mg / ml MTT was added to each well, and incubated at 37 °C for 4 h. The absorbance at 570 nm was detected by a multifunctional microplate reader (GeniosPro, Tecan, US). The survival rate of the cells was used as an indicator of the toxicity of the compound SSX to MDCK cells.
[0049] Cell viability (%)=E / N×100
[0050] E is the absorbance of the drug group, and N is the absorbance of the cell control group.
[0051] Test results: Compound SSX has less cytotoxicity, CC 50...
Embodiment 3
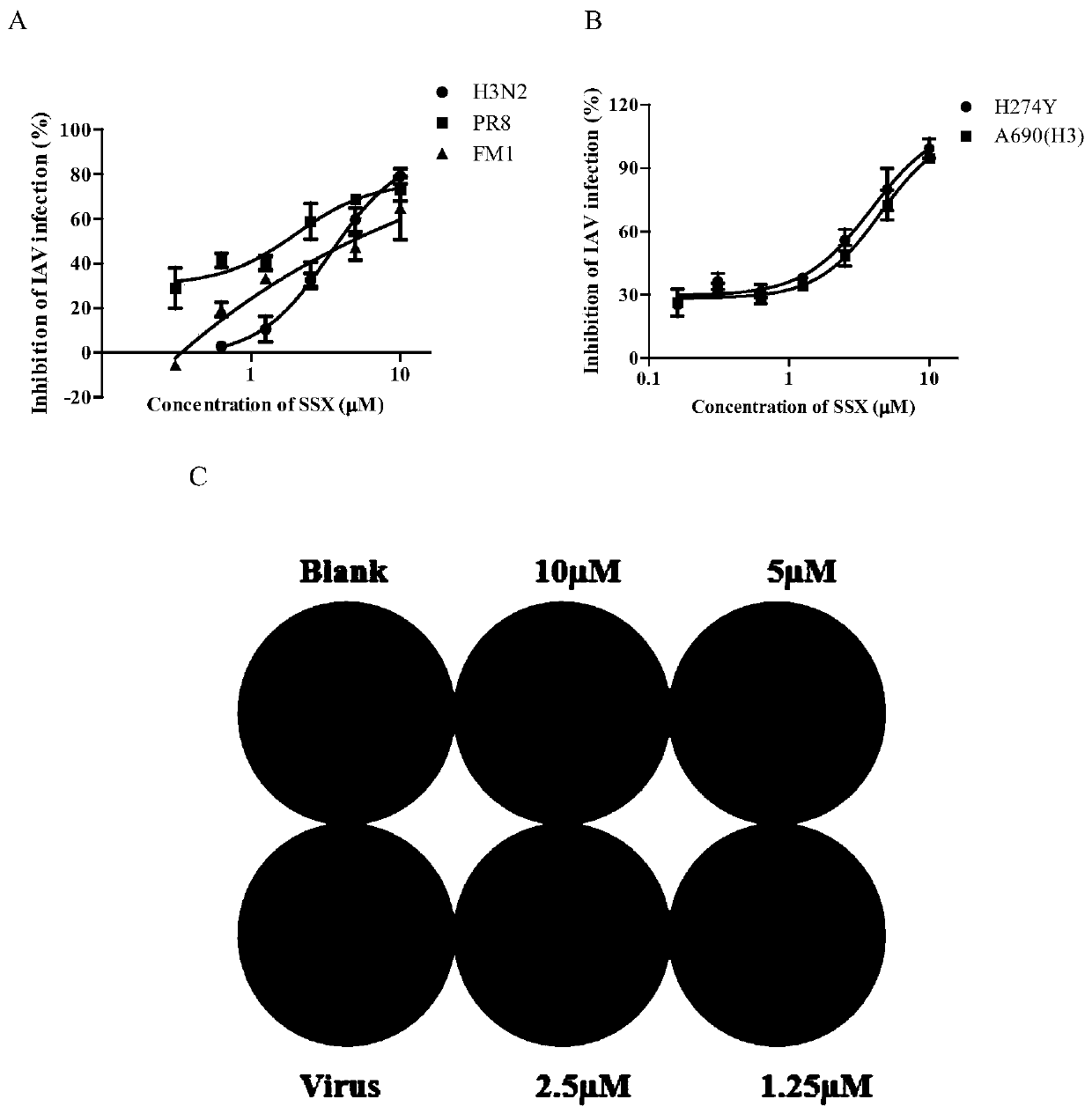
[0053] The inhibitory activity of embodiment 3 compound SSX to various subtype influenza viruses
[0054] In vitro antiviral experiments of the present invention involve multiple subtypes of influenza A viruses, including A / Aichi / 2 / 68 (H3N2), A / FM-1 / 1 / 47 (H1N1), A / Puerto Rico / 8 / 34(H1N1), H274Y and A 690(H3), the specific method is as follows:
[0055] MDCK cells by 2 x 10 4 / well seeded in 96-well plate at 37°C, 5% CO 2 cultured to a monolayer in a constant temperature cell incubator. Use 100TCID 50 Infect the cells with influenza A virus, 100 μl per well, incubate at 37°C for 1 hour, discard the virus solution, add 200 μl of compound SSX serially diluted in DMEM (containing 1 μg / ml TPCK), and continue to incubate for 48 hours. Combining the MTT method and the plaque experiment, reflecting the antiviral activity of the compound SSX is determined by the protective effect of the compound SSX on cells, including observing the cell virus phenomenon (CPE) caused by the compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com