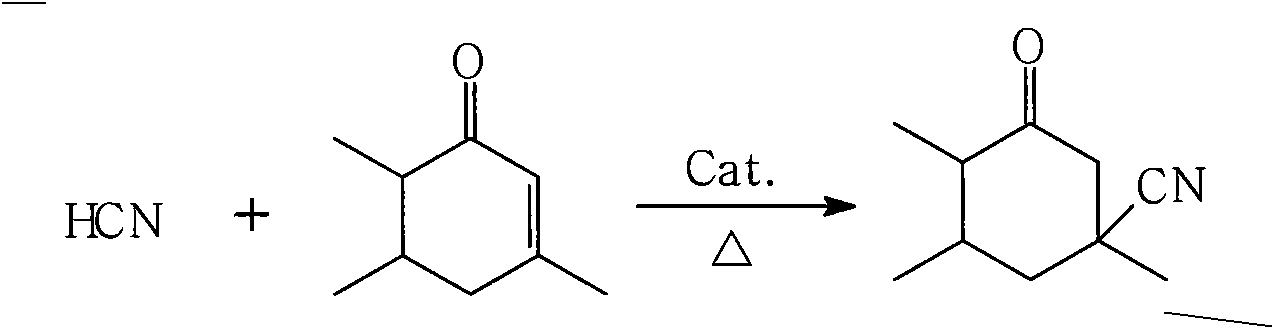
Isophorone nitrile production method using acrylonitrile byproduct hydrocyanic acid continuous reaction
A technology of isophorone nitrile and isophorone, which is applied in the field of producing isophorone nitrile, can solve the problems of unrecoverable activity, increased production cost, energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Catalyst preparation:
[0036] In a 1000ml beaker, add 500ml of 10A molecular sieve, then add 500ml of pre-configured calcium chloride solution with a content of 25%, stir several times with a glass rod at intervals of ten minutes, soak for 2 hours, filter, and remove an appropriate amount Rinse with deionized water, then add 500ml of pre-configured saturated sodium bicarbonate solution, stir several times with a glass rod at intervals of ten minutes, soak for 2 hours, filter, rinse with appropriate amount of deionized water, and then put it in the oven After drying at 90°C for 4 hours to constant weight, transfer it to a muffle furnace, calcine at 900°C for more than 3 hours, take it out, and put it in a desiccator to cool down for later use.
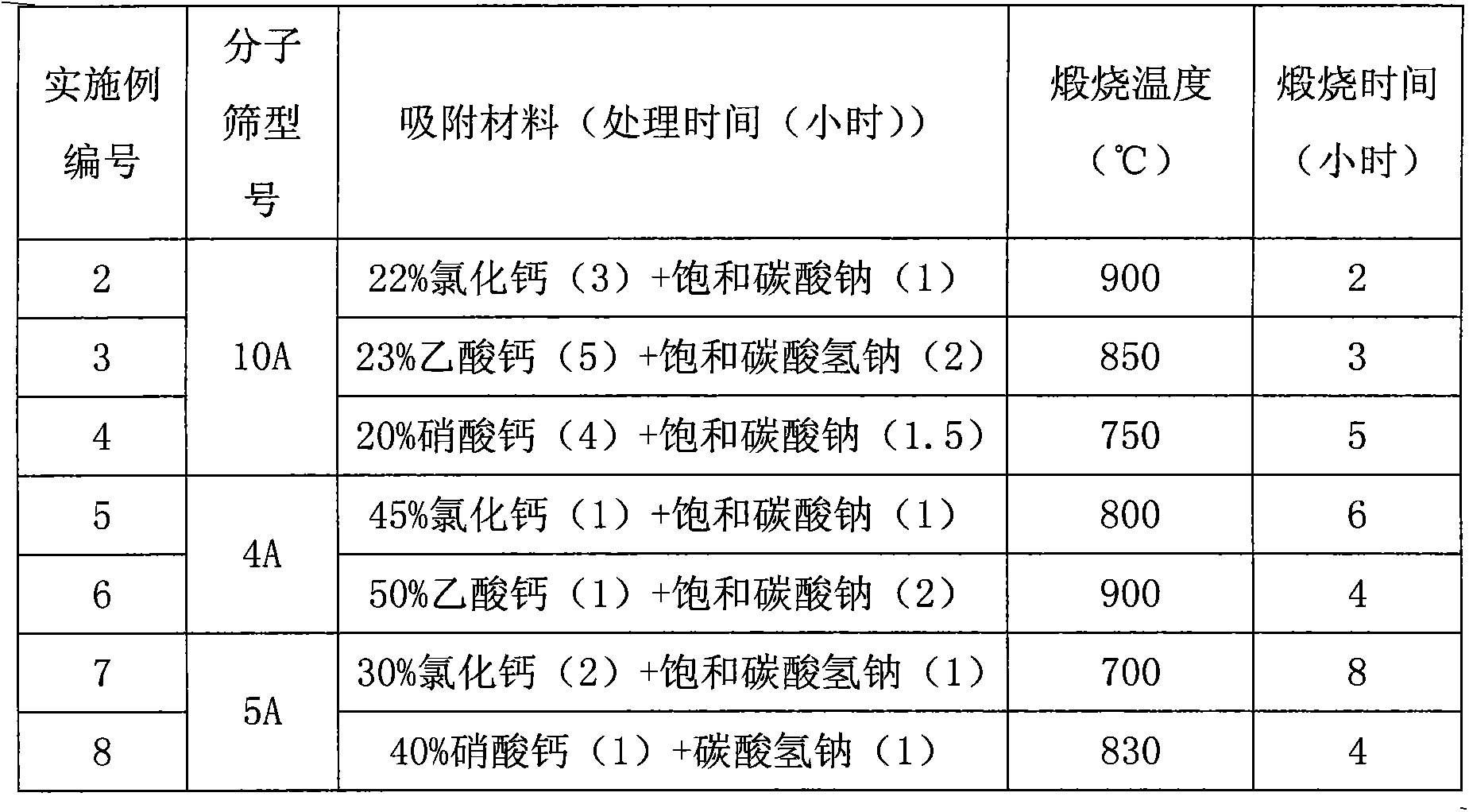
Embodiment 2-8
[0038] Obtain method according to embodiment 1, change processing condition, prepare corresponding molecular sieve catalyst:
[0039]
[0040] Example 1:
[0041] Continuous reaction to prepare isophorone nitrile:
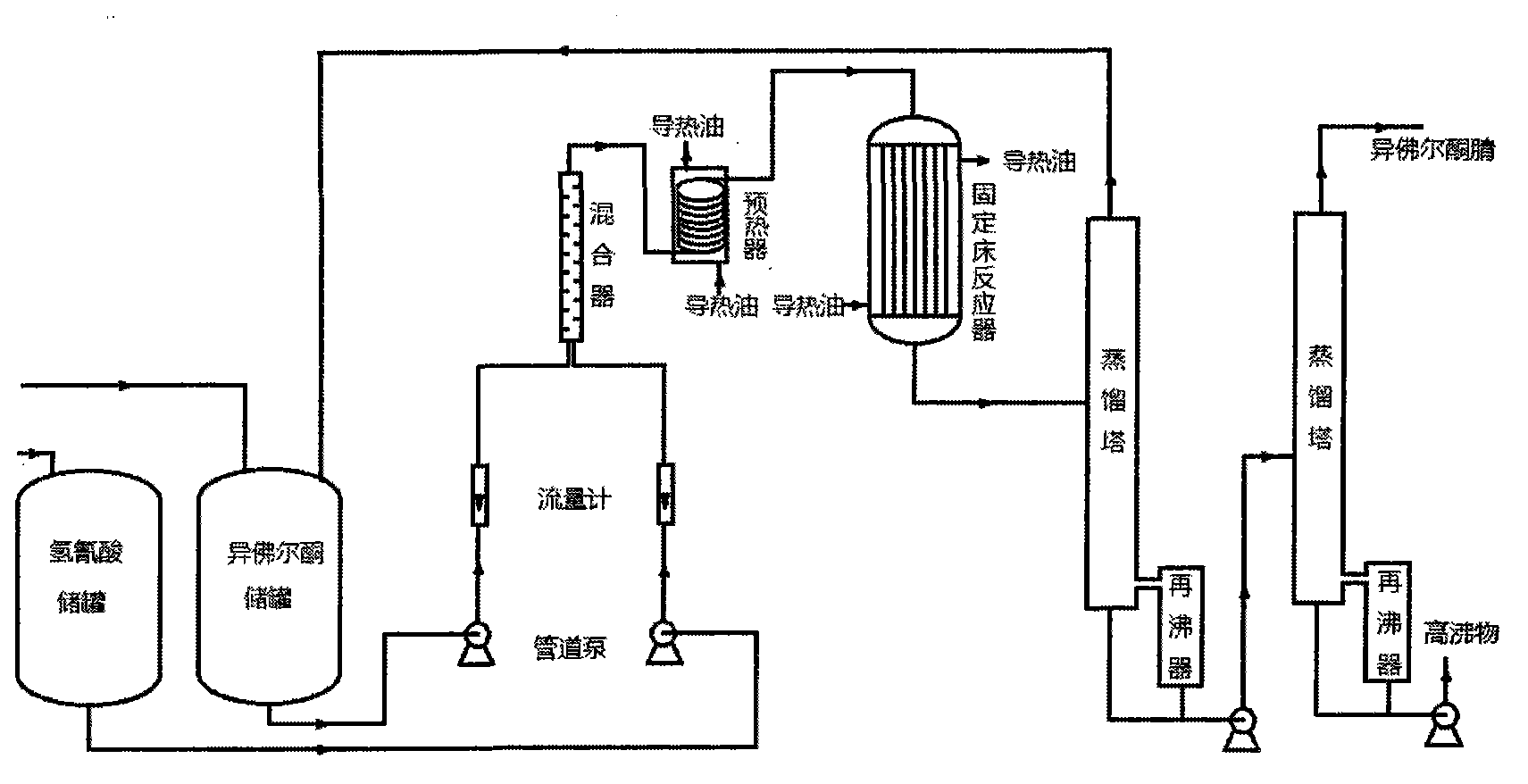
[0042]In the fixed-bed reactor, fill the catalyst of Example 1 made according to the above-mentioned process, turn on the isophorone pump, and adopt a larger flow rate to make the raw material isophorone fill the preheater, pipeline and fixed-bed reactor and circulate Return to the storage tank, adjust the flow rate of isophorone to 12ml / min at this time, open the heat transfer oil valve of the preheater, preheat the raw material of isophorone to the set temperature of 100°C, and open the heat transfer oil valve of the fixed bed reactor to make the fixed bed reactor The bed reached the set reaction temperature of 130°C. When the equipment is debugged and stabilized, turn on the hydrocyanic acid pipeline pump, adjust the flow rate of hydrocyanic acid to 1ml / min...
Embodiment 2
[0043] Example 2: In the fixed-bed reactor, fill the catalyst of Example 2 made according to the above-mentioned process, turn on the isophorone pump, and use a larger flow rate to make the raw material isophorone fill the preheater, pipeline and fixed bed The reactor is circulated back to the storage tank. At this time, adjust the flow rate of isophorone to 13ml / min, open the heat transfer oil valve of the preheater, preheat the isophorone raw material to the set temperature of 80°C, and turn on the heat transfer oil of the fixed bed reactor. valve to make the fixed bed reach the set reaction temperature of 180°C. When the equipment is debugged and stabilized, turn on the hydrocyanic acid pipeline pump, adjust the flow rate of hydrocyanic acid to 2ml / min, control the volume ratio of hydrocyanic acid to isophorone at 10:1, and the hydrocyanic acid and isophorone pass through the mixer Finally, stay in the preheater for a short time (10 seconds), preheat to the set temperature,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com