Polybasic layered oxide lithium ion battery material and preparation method thereof
A technology for lithium-ion batteries and battery materials, which is applied in the field of multi-layered oxide lithium-ion battery materials and its preparation, can solve the problems of expensive Co element and high battery cost, and achieve good reversibility of charge and discharge, cycle Improved performance and good cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
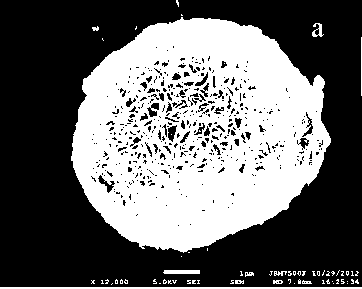
[0027] 1) Preparation (Ni 0.8 co 0.1 mn 0.1 )(OH) 2 : Weigh 0.00512mol Ni(NO 3 ) 2 ·6H 2 O, 0.00064mol Co(NO 3 ) 2 ·6H 2 O, 0.00064mol Mn(NO 3 ) 2 and 0.0064mol urea were dissolved in 64 mL solvent (ethanol: water = 4:1), and the corresponding concentrations of nickel, cobalt, and manganese in the solution obtained after mixing were 0.08, 0.01, and 0.01 mol / L, respectively. Then the mixed solution was transferred to a 100mL reaction kettle, reacted at 180°C for 7.5 hours, naturally cooled to room temperature, centrifuged, and dried at 60°C for 12 hours to obtain the precursor (Ni 0.8 co 0.1 mn 0.1 )(OH) 2 .
[0028] 2) Disperse 20mL of 0.00673mol / L aluminum nitrate solution with 0.25g (Ni 0.8 co 0.1 mn 0.1 )(OH) 2 After fully mixing the 30m aqueous solution, transfer it to a 90 mL reaction kettle and react at 150°C for 4 hours, naturally cool to room temperature, centrifuge, and dry at 60°C to obtain a precursor with an optimized composition. Its molecular f...
Embodiment 2
[0034] (Ni 0.8 co 0.1 mn 0.1 )(OH) 2 The preparation is with embodiment 1.
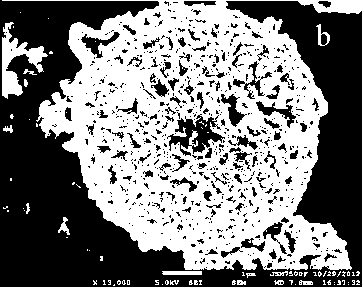
[0035] 20mL of 0.00673mol / L aluminum acetate solution and dispersed with 0.25g (Ni 0.8 co 0.1 mn 0.1 )(OH) 2 Mix 30mL of aqueous solution of the solution and transfer it to a 90mL reactor for 4 hours at 120°C for 4 hours, naturally cool to room temperature, centrifuge, and dry at 60°C to obtain a precursor with optimized composition. Then it is fully mixed with lithium hydroxide in a stoichiometric ratio, and then calcined at 480° C. for 5 hours and 650° C. for 12 hours respectively to obtain a multi-layered oxide lithium ion battery material.
[0036] The lithium-ion battery material with optimized composition prepared by the above steps was made into a bonded electrode for charge and discharge tests. The results showed that the discharge specific capacity of the prepared multi-layered oxide lithium-ion battery material was about 160 mAh / g.
Embodiment 3
[0038] (Ni 0.8 co 0.1 mn 0.1 )(OH) 2 The preparation is with embodiment 1.
[0039] The aluminum nitrate solution of 20mL 0.00673mol / L and the precursor (Ni 0.8 co 0.1 mn 0.1 )(OH) 2 30mL of the aqueous solution was fully mixed and then transferred to a 90mL reactor to react at 160°C for 4 hours, naturally cooled to room temperature, centrifuged, and dried at 60°C to obtain a precursor with optimized composition. Then, it is fully mixed with lithium hydroxide in a stoichiometric ratio, and then calcined at 500° C. for 5 hours and 650° C. for 12 hours respectively to obtain a multi-layered oxide lithium ion battery material.
[0040] The lithium-ion battery material with optimized composition prepared by the above steps was made into a bonded electrode for charge and discharge tests. The results showed that the discharge specific capacity of the prepared multi-layered oxide lithium-ion battery material was about 160 mAh / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com