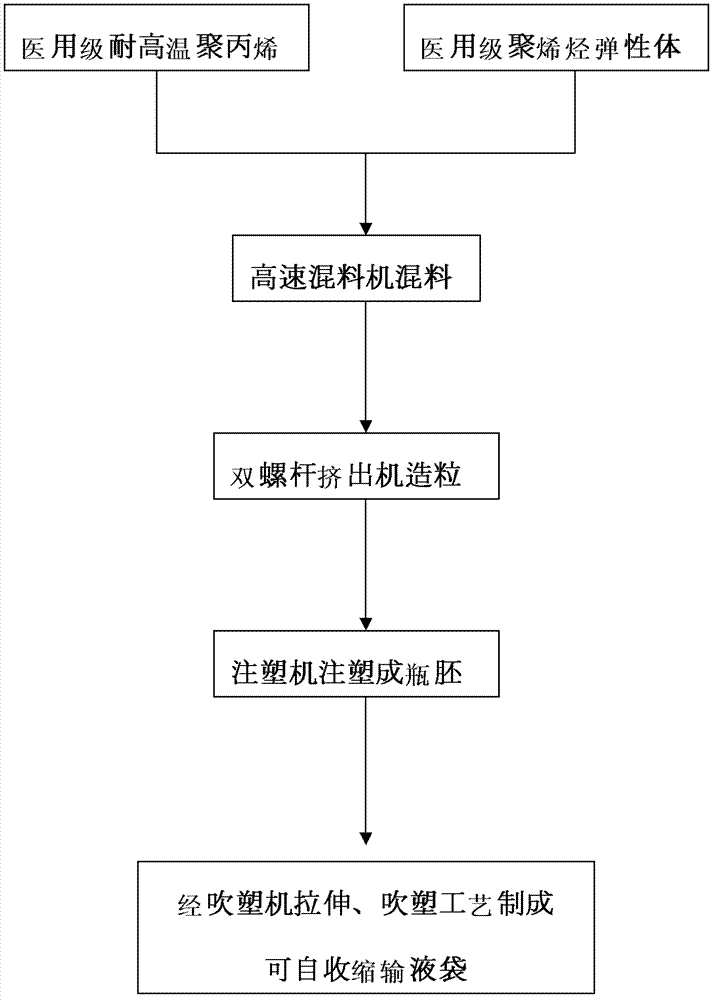
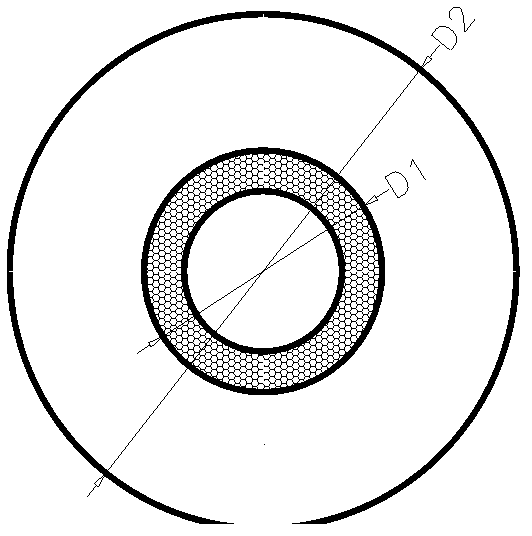
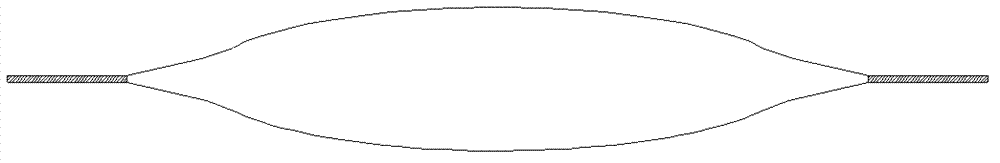
Transfusion bag with self-contraction function and transfusion bag special material thereof
A technology for infusion bags and special materials, which is applied in the field of infusion bags and special materials for infusion bags, which can solve the problems of high risk of leakage, achieve good toughening effect, reduce the risk of leakage, and improve the effect of dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0039] Experimental Example 1: Self-shrinkage effect detection of the present invention
[0040] The infusion bag of the present invention (prepared in Example 1), the polypropylene infusion bottle, and the three-layer co-extruded film infusion bag of the same specification are filled with sodium chloride injection (100ml, 0.9g) respectively, and 10 bags (bottles) are each taken Carry out the liquid discharge completeness test at a height of 1.2m from the ground. During the liquid discharge process, do not insert the vent needle or close the vent hole of the infusion set, and make the flow rate regulator of the infusion set fully open, and the infusion needle completely penetrates the combination. When the infusion is stopped, it is considered as the end point of the discharge. At this time, the infusion needle is pulled out and the sodium chloride injection remaining in the bag (bottle) is taken out and quantified with a syringe. The better the self-shrinking effect, the smal...
experiment example 2
[0043] Experimental example two, the present invention leaks bag rate detection after transportation test:
[0044] The infusion bag of the present invention (prepared in embodiment one) of the same specification, the polypropylene infusion bottle, and the three-layer co-extrusion film infusion bag are filled with sodium chloride injection (100ml, 0.9g) respectively, and in a corrugated box of the same specification The above samples were placed neatly respectively, 100 bags (bottles) per case, 40 cases per layer, 5 layers in total, 200 cases in total, and the above three samples were respectively placed in the car for long-distance transportation test.
[0045] The samples were sent from Xindu District, Chengdu to Urumqi, Xinjiang from November 15, 2010, and returned immediately after arriving, and returned to Xindu District, Chengdu, where the company is located, on December 8, 2010. The round trip took about 24 days in total.
[0046]Detection method: After transportation, ...
experiment example 3
[0050] Experimental example three, the biological inspection of the transfusion bag of the present invention
[0051] The infusion bag of the present invention (prepared in Example 1) was tested according to the United States Pharmacopoeia USP32 and the standards of YBB00012003, YBB00032003, and YBB00052003. The test results met the requirements of the above standards. The test results are shown in Table 3.
[0052] Table 3, biological test report of the present invention
[0053] serial number
[0054] 2
[0055] The following experimental examples and examples are used to further illustrate but not limit the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com