Method for separating nickel-cobalt and manganese in nickel-cobalt-manganese material with high manganese-cobalt ratio
A nickel-cobalt-manganese and nickel-cobalt-manganese lithium technology, which is applied in the fields of hydrometallurgy and chemical production, can solve the problems of low nickel-cobalt recovery rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
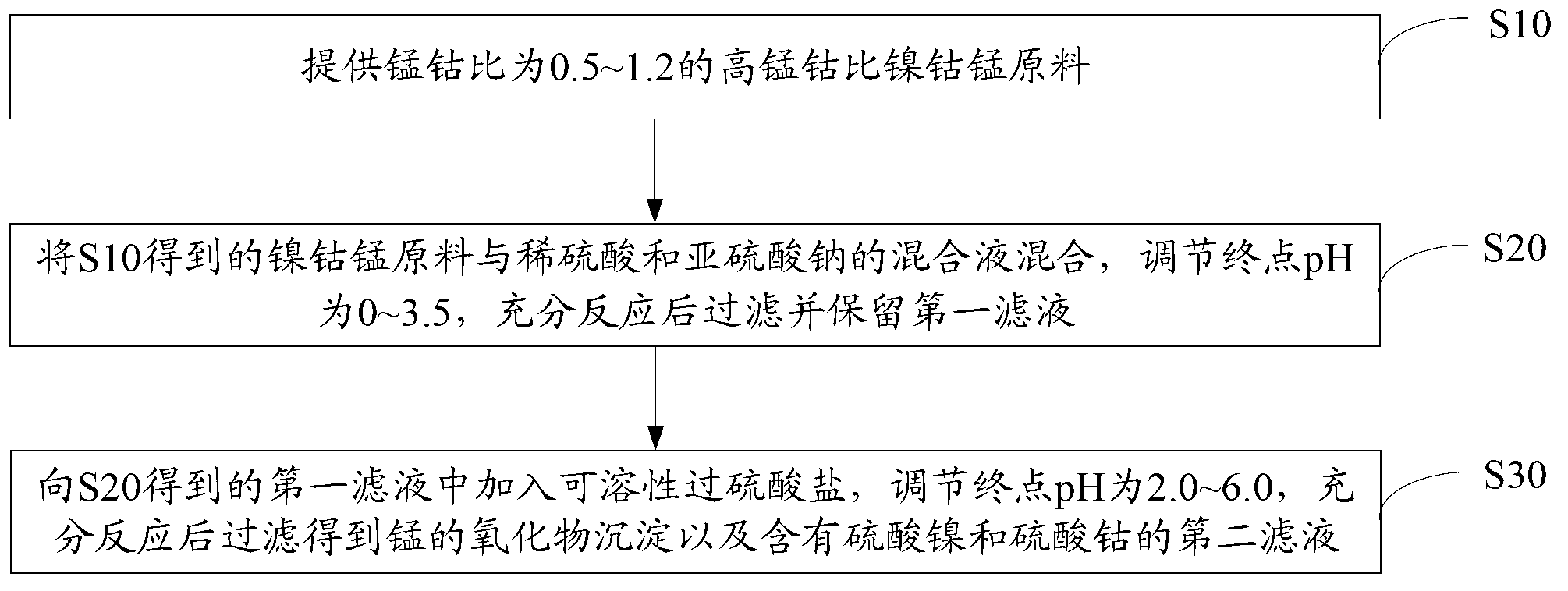
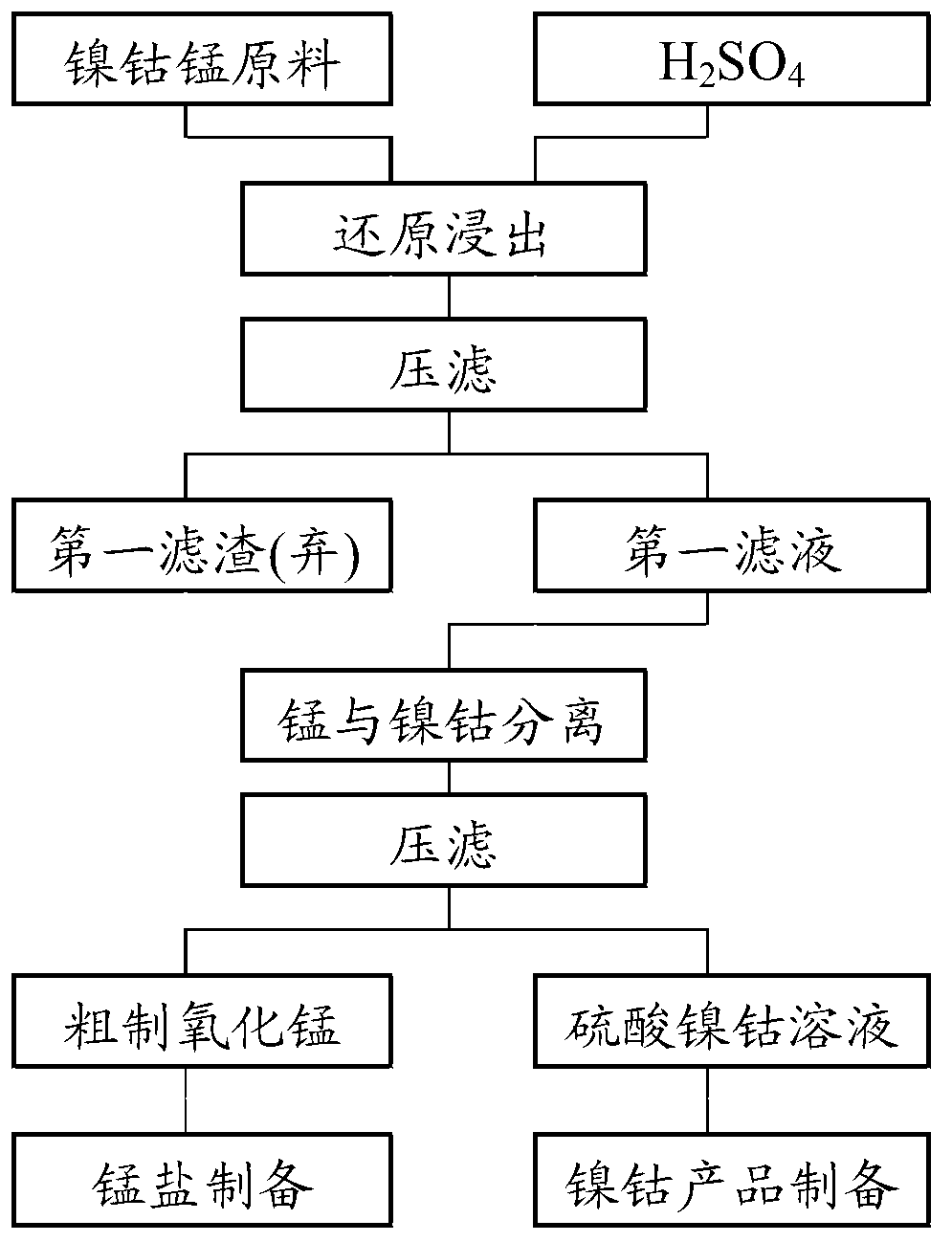
[0058] Cobalt nickel manganese raw material (Co 13.8%, Ni 12.9%, Cu 0.36%, Mn 10.16%, Zn 1.23%, Fe1.15%, Pb 0.21%, Si 0.13%, Ca 0.21%, Mg 0.66%), with H 2 SO 4 +Na 2 SO 3 (Na 2 SO 3 1.5~1.6 times the theoretical amount) leaching, liquid-solid ratio 8kg / 1kg, temperature 75°C, stirring time 2.5h, Na 2 CO 3 As a neutralizing agent, the end point pH is 1.5. The leaching recovery rate of cobalt was 98.71%, the leaching recovery rate of nickel was 99.12%, and the leaching recovery rate of manganese was 98.76%.
[0059] Na 2 S 2 o 8 (1.6 times the theoretical amount) is an oxidizing agent, Na 2 CO 3 As a neutralizing agent, the temperature is 75°C, the stirring time is 1.5hr, and the final pH is 4.5 to precipitate and remove manganese. The precipitation removal rate of manganese was 99.32%, and the leaching recovery rate of cobalt was 99.13%. The leaching recovery rate of nickel was 99.53%.
[0060] After the cobalt and manganese are separated, the nickel-cobalt sulfate...
Embodiment 2
[0062] Waste nickel-cobalt-manganese ternary secondary battery cathode material (Li 6.99%, Co 4.82%, Ni 12.11%, Cu 0.01%, Mn 6.61%, Zn 0.02%, Fe 0.06%, Al 3.75%, Ca 0.001%, Mg 0.001 %), with H 2 SO 4 +Na 2 SO 3 (Na 2 SO 3 1.5~1.6 times the theoretical amount) leaching, liquid-solid ratio 6kg / 1kg, temperature 75°C, stirring time 2.5h, Na 2 CO 3 As a neutralizing agent, the end point pH is 1.5. The leaching recovery rate of cobalt was 98.68%, the leaching recovery rate of nickel was 99.33%, and the leaching recovery rate of manganese was 98.71%.
[0063] For leachate (NH 4 ) 2 S 2 o 8 (1.6 times the theoretical amount) is an oxidizing agent, Na 2 CO 3 As a neutralizing agent, the temperature is 75°C, the stirring time is 1.5hr, and the final pH is 4.5 to precipitate and remove manganese. The precipitation removal rate of manganese was 99.63%, and the leaching recovery rate of cobalt was 98.18%. The leaching recovery rate of nickel is 98.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com