Tablet containing everolimus and preparation method thereof
A technology for everolimus and tablets, which is applied in the field of everolimus-containing tablets and its preparation, can solve problems affecting product properties and potential safety hazards, and achieve convenient operation, improved safety, and improved hydrophilicity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
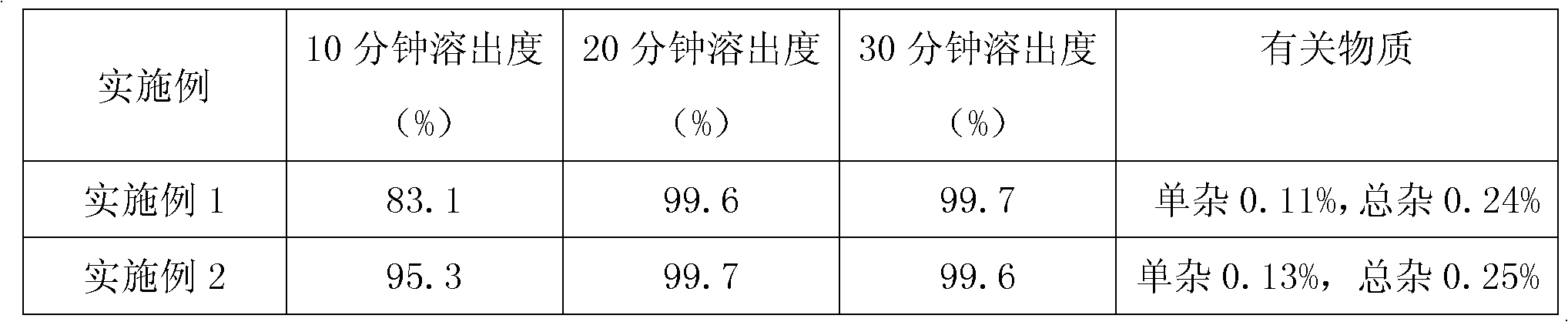
Examples
Embodiment 1
[0032] 1.1 Preparation of everolimus and crospovidone mixture
[0033] Everolimus 1 part
[0034] 4 parts absolute ethanol
[0035] Crospovidone 0.5 parts
[0036] Preparation process: after dissolving everolimus in absolute ethanol, add to crospovidone, and dry under reduced pressure to obtain mixture A;
[0037] 1.2 Preparation of Everolimus Tablets
[0038] Mixture A 1 part
[0039] Lactose 80 parts
[0040] 1 part magnesium stearate
[0041] Mix the mixture A with lactose and magnesium stearate, and press into tablets.
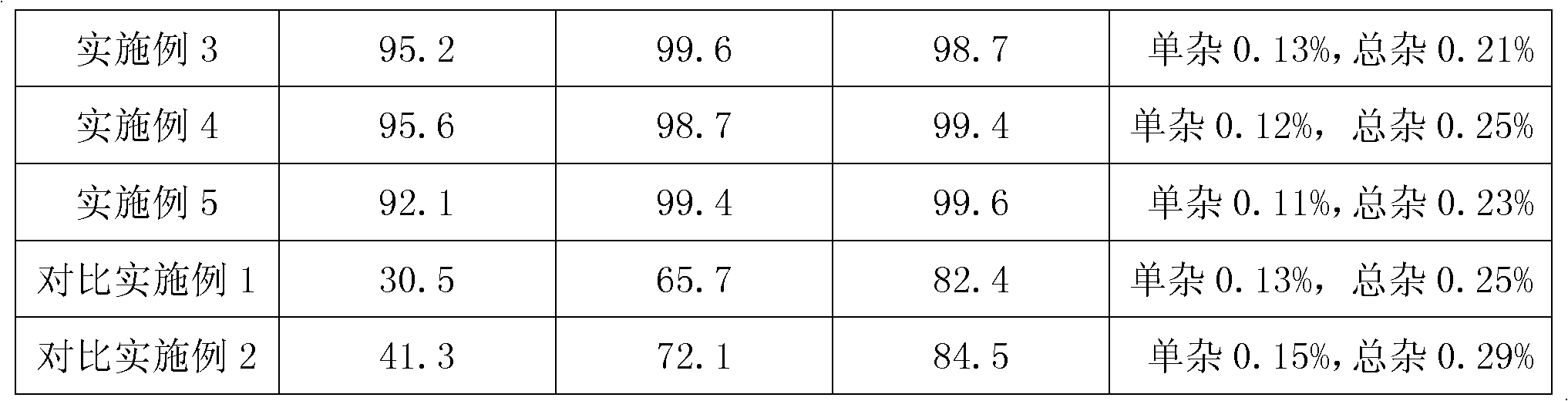
Embodiment 2
[0043] 2.1 Preparation of everolimus and crospovidone mixture
[0044] Everolimus 1 part
[0045] 400 parts of absolute ethanol
[0046] Crospovidone 100 parts
[0047] Preparation process: after dissolving everolimus in absolute ethanol, add to crospovidone, and dry under reduced pressure to obtain mixture A;
[0048] 2.2 Preparation of Everolimus Tablets
[0049] Mixture A 1 part
[0050] 1 part lactose
[0051] Magnesium stearate 0.01 parts
[0052] Mix the mixture A with lactose and magnesium stearate, and press into tablets.
Embodiment 3
[0054] 3.1 Preparation of everolimus and crospovidone mixture
[0055] Everolimus 1 part
[0056] Chloroform 80 parts
[0057] 30 parts of crospovidone
[0058] Preparation process: after dissolving everolimus in chloroform, adding to crospovidone, drying under reduced pressure to obtain mixture A;
[0059] 3.2 Preparation of Everolimus Tablets
[0060] Mixture A 1 part
[0061] Mannitol 0.8 parts
[0062] Talcum powder 0.05 parts
[0063] Mix the mixture A with lactose and magnesium stearate, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com