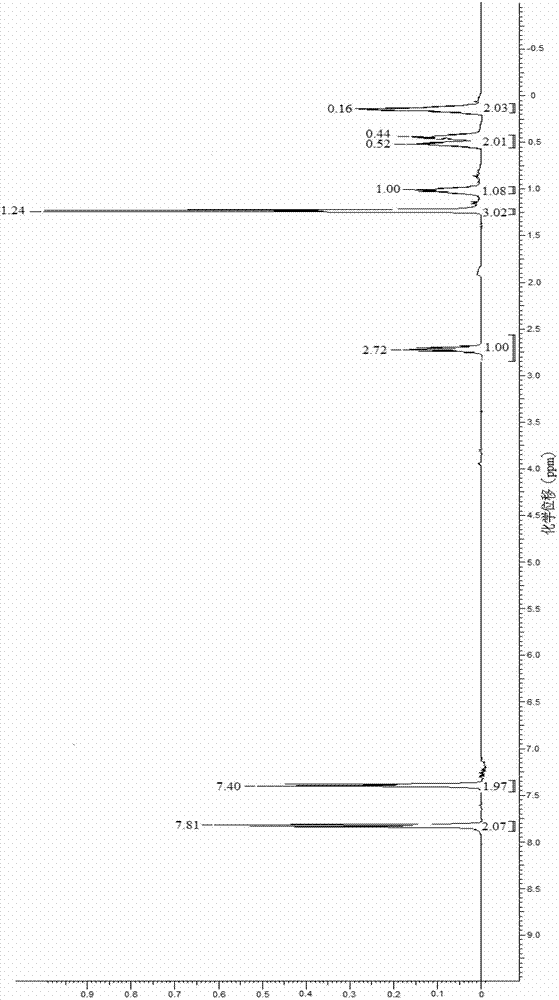
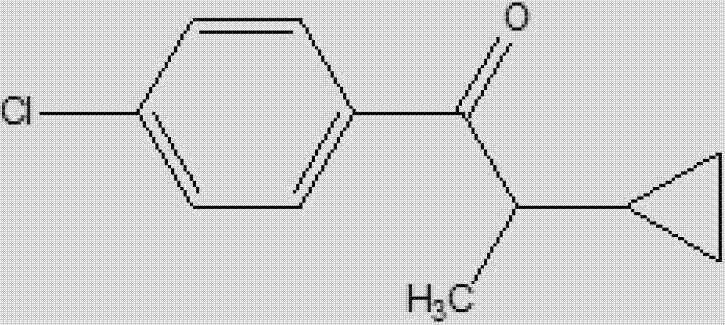
Synthetic method for 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
A synthesis method, cyclopropyl technology, applied in the direction of condensation preparation of carbonyl compounds, organic chemistry, etc., can solve the problems of long production cycle, high cost consumption, and many operational steps, and achieve low cost, less harsh reaction conditions, and high efficiency. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0014] Specific embodiment one: the synthetic method of the 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone of the present embodiment is carried out according to the following steps:
[0015] One, configuration sulfur ylide reagent: according to the molar ratio of thioether and dimethyl sulfate is (1~3): 1, according to the molar ratio of dimethyl sulfate and tert-butanol is (1~1.5): 1, first Add tert-butanol into thioether, stir for 20min-40min at a temperature of 25-35°C and a stirring speed of 80r / min-150r / min, then add dimethyl sulfate dropwise, and continue to The temperature is 25-35°C, the stirring speed is 80r / min-150r / min, stirring until the solution is white and viscous, and the sulfur ylide reagent is obtained;
[0016] Two, epoxidation reaction: be 1: (3~5) by the mass ratio of cyclopropyl methyl ketone and toluene, be 1: (1~5) by the mole of the sulfur ylide reagent of cyclopropyl methyl ketone and step one 3), by the mol ratio of cyclopropyl methyl ketone and potas...
specific Embodiment approach 2
[0023] Specific embodiment two: what this embodiment is different from specific embodiment one is: the mole of thioether and dimethyl sulfate is 2: 1 in the step 1, by the mol ratio of dimethyl sulfate and tert-butyl alcohol (1.2~1.3 ): 1, other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0024] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that in step one, tert-butanol is first added to the thioether, at a temperature of 30°C and a stirring speed of 80r / min to 150r / min Under the conditions, stir for 30 minutes, then add dimethyl sulfate dropwise, after the dropwise addition, continue to stir at a temperature of 30°C and a stirring speed of 80r / min to 150r / min until the solution is white and viscous, and the sulfur ylide reagent is obtained. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com