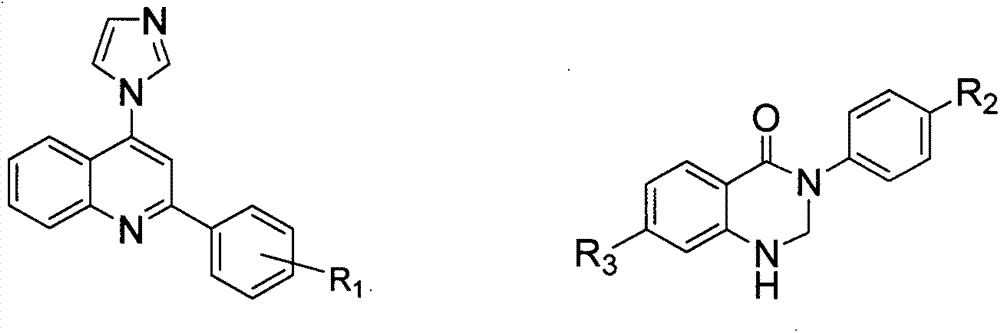
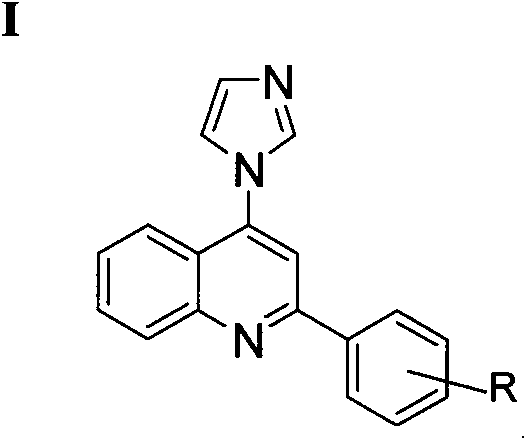
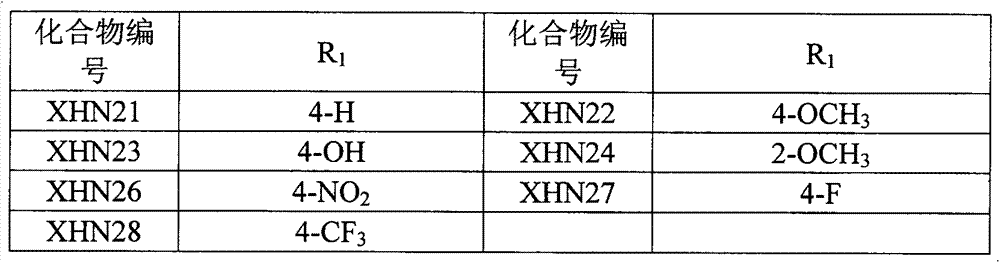
4-imidazolyl quinoline and quinazoline ketone aromatizing enzyme inhibitors as well as preparation method and medical application thereof
An alkyl, pharmaceutical technology, applied in the field of 4-imidazolyl quinoline and quinazolinone aromatase inhibitors, prevention or treatment of estrogen maintenance diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0051] The specific embodiment (described embodiment is only used to illustrate the present invention, is not used to limit the present invention)
preparation example
[0052] The preparation examples of some compounds are as follows:
[0053] Melting point uses XT4 microscopic melting point measuring instrument; NMR spectrometer is Bruker AV500 type (TMS is internal standard); mass spectrometer is Shimadzu GCMS-QP2010 mass spectrometer or Mariner mass spectrometer; infrared spectrometer is Nicolet Impact410 type (KBr Tablets); the elemental analyzer was Elementar Vario EL III.
[0054] The silica gel used for column chromatography is 100-200 mesh, 200-300 mesh or 300-400 mesh silica gel (Qingdao Ocean Chemical Factory), and the eluent is petroleum ether-ethyl acetate system or chloroform-methanol system. GF for thin layer chromatography (TLC) 254 Thin-layer chromatography plate (Yantai Jiangyou Silica Gel Development Co., Ltd.); TLC development system is petroleum ether-ethyl acetate system or chloroform-methanol system, and a small amount of acetic acid is added if necessary; TLC is performed on a ZF7 type three-purpose ultraviolet analyze...
Embodiment 1
[0057] Preparation of o-aminoacetophenone (1)
[0058] Put 1.52g (9.21mmol) o-nitroacetophenone in the reaction flask, add 100mL THF and 50mL water solution, add 5g (92.1mmol) reduced iron powder, drop in 9 drops of concentrated hydrochloric acid, heat up to 70°C, reflux Reaction 2h. TLC monitors that the reaction is complete, stop heating, cool to room temperature, filter to remove iron powder, distill the filtrate under reduced pressure to remove THF, extract with ethyl acetate, combine organic phases, wash with water, wash with saturated brine, and anhydrous NaSO 4 Dry overnight, and distill to dryness under reduced pressure to obtain about 1.13 g of light yellow oily o-aminoacetophenone (1), with a yield of 91%. ESI-MS: m / z=136[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com