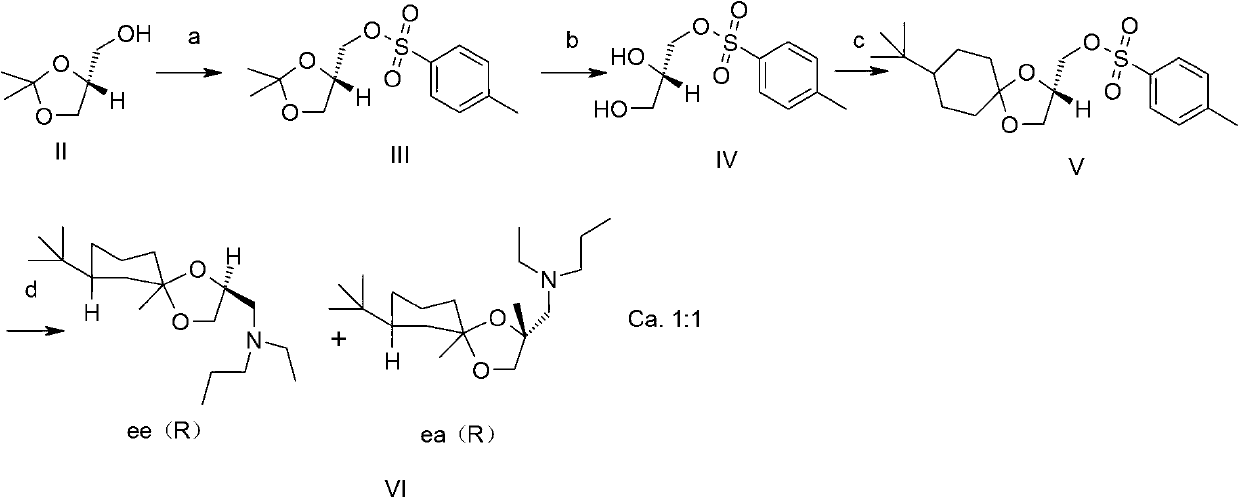
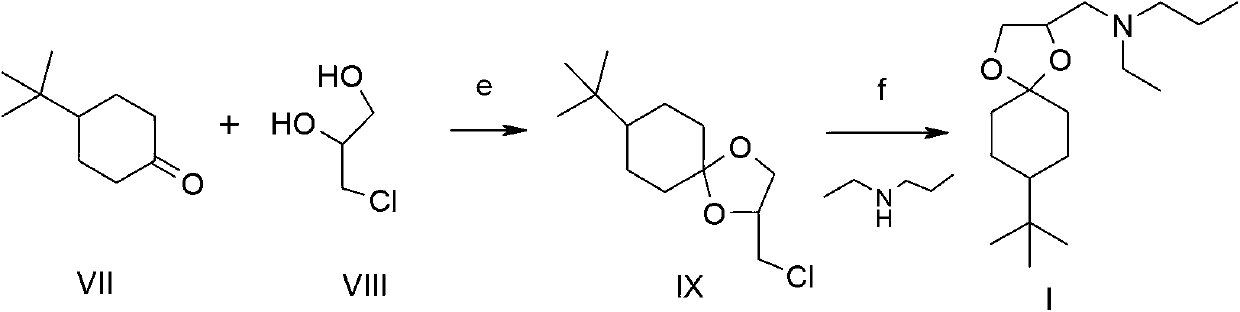
Synthesis method of spiroxamine as bactericide
A synthesis method and spirocycline technology are applied in the field of fungicides synthesis, which can solve the problems of expensive raw materials, difficult purification of intermediates, and inability to produce large-scale production, and achieve high reaction yield, low cost, and mild experimental conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
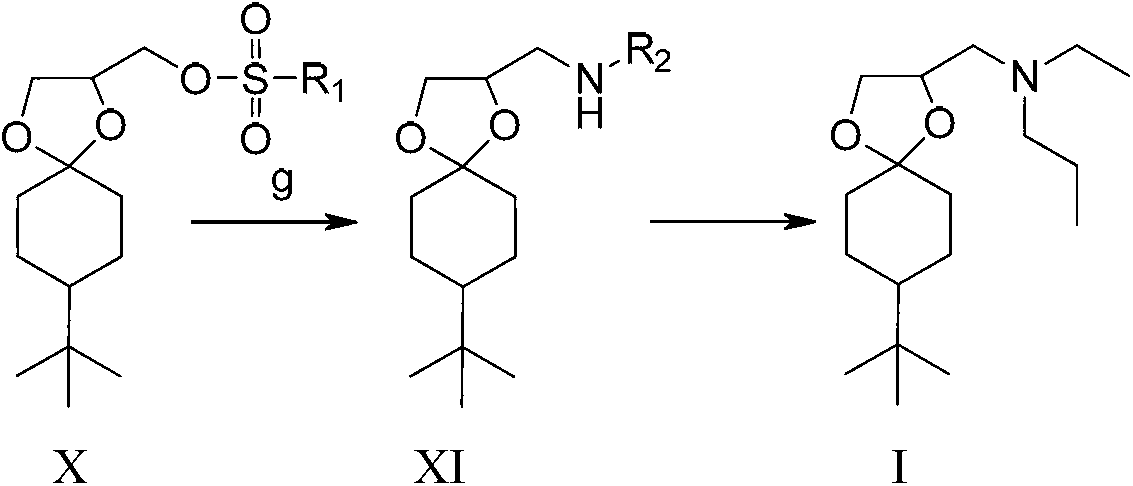
[0032] (1) Synthesis of compound N-ethyl-8-tert-butyl-1,4-dioxaspiro[4.5]decane-2-methylamine XI:
[0033]
[0034] 50 grams of {8-tert-butyl-1,4-dioxaspiro[4.5]decane-2-methyl} methanesulfonate X (0.163 mol), 21.9 grams of 67% Ethylamine aqueous solution (0.326 mol) and 24.8 grams of potassium carbonate (0.179 mol), then heated to 110-115 ° C, the internal pressure is 0.4MPa stirring reaction after 24 hours, the reaction mixture was distilled to remove ethanol and excess ethylamine aqueous solution recovery, The residue was added with 700 ml of dichloroethane and 300 ml of water, stirred and dissolved, and allowed to stand for stratification. The organic layer was concentrated under reduced pressure to obtain 39.5 g of brown liquid N-ethyl-8-tert-butyl-1,4-dioxaspiro [4.5] Decane-2-methylamine XI, yield 94.9%. 1 H NMR (400MHz, CDCl3) δ (ppm) 4.20-4.30 (m, 1H), 4.00-4.10 (m, 1H), 3.6-3.7 (m, 1H), 2.60-2.80 (m, 4H), 1.80-1.90 (m,1H),1.70-1.80(m,3H),1.60(m,1H),1.2-1.5(m,3H)...
Embodiment 2
[0039] (1) Synthesis of compound N-propyl-8-tert-butyl-1,4-dioxaspiro[4.5]decane-2-methylamine XII:
[0040]
[0041] In a 500 ml autoclave, 25.0 g of {8-tert-butyl-1,4-dioxaspiro[4.5]decane-2-methyl} ester X (81.6 mmol) and 9.65 g of n- Propylamine (163.2 mmol), 12.4 grams of potassium carbonate (89.76 mmol) and 150 milliliters of ethanol, the reaction mixture was heated to 110-115 ° C and the internal pressure was 0.5 MPa, reacted under stirring for 24 hours and then cooled to room temperature, the reaction mixture was distilled off Ethanol and excess propylamine were recovered and applied mechanically. The residue was added with 500 ml of dichloroethane and 200 ml of water, stirred and dissolved, and left to stand for layering. The organic layer was concentrated under reduced pressure to obtain 17.6 g of brown oil N-propyl-8-tert-butyl -1,4-dioxaspiro[4.5]decane-2-methylamine XII, the yield is 80%. 1H NMR (400MHz, CDCl3) δ (ppm) 4.20-4.30 (m, 1H), 4.01-4.06 (m, 1H), 3.6...
Embodiment 3
[0046] (1) Synthesis of compound N-propyl-8-tert-butyl-1,4-dioxaspiro[4.5]decane-2-methylamine XII
[0047]
[0048] 31.2 grams of p-toluenesulfonic acid {8-tert-butyl-1,4-dioxaspiro[4.5]decane-2-methyl} ester X' (81.59 mmol), 14.47 grams n-Propylamine (244.76 mmol), 12.4 g of potassium carbonate (89.75 mmol) and 150 ml of ethanol were heated in an autoclave to 118-120 °C with an internal pressure of 0.7 MPa. After stirring for 24 hours, concentrate under reduced pressure to recover ethanol and excess isopropylamine, add 700 milliliters of dichloroethane and 500 milliliters of water to the residue, stir and stand for stratification, and concentrate the organic phase under reduced pressure to obtain 21 grams of N-propyl-8-tert-butyl-1,4 -Dioxaspiro[4.5]decane-2-methylamine XII, yield: 95.5%. 1 H NMR (400MHz, CDCl3) δ (ppm) 4.20-4.30 (m, 1H), 4.01-4.06 (m, 1H), 3.65-3.67 (m, 1H), 2.70-2.74 (m, 2H), 2.57-2.63 (q,2H),1.10-1.90(m,10H),1.00(m,1H),0.90(m,3H),0.80-0.90(m,9H).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com