Ertapenem pharmaceutical composition
A technology of ertapenem and composition, which is applied in the pharmaceutical composition of carbapenem antibiotics and its preparation field, can solve problems such as insufficient stability, and achieve the effects of improving stability and preventing polymerization and hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 1.0 g each of hydroxyethyl starches with an average molecular weight of 130,000 (molar substitution level is 0.4) and 200,000 (molar substitution level is 0.5), add water to dissolve to 10 ml, and place them in a refrigerator at 4°C to cool for later use. Weigh 2.25g of ertapenem raw material (the content is 99.1% according to HPLC analysis, which is expressed as the content of the raw material below. For specific analysis methods, please refer to Sajonz P, Vailaya A, Sudah O, McPherson L, et al. Development of a gradient elution preparative high performance liquid chromatography method for the recovery of the antibiotic ertapenem from crystallization process streams. J Chromatogr A. 2006; 1126: 365-372.), add 10ml of 4°C water to dissolve. Use 2mol / L NaOH to adjust the pH of the solution to 7.3, then add a small amount of 4°C water to make the volume 12ml and shake well. Pipette 4ml of ertapenem solution into 2 vials, then add 1ml of the above 2 kinds of 10% hydr...
Embodiment 2
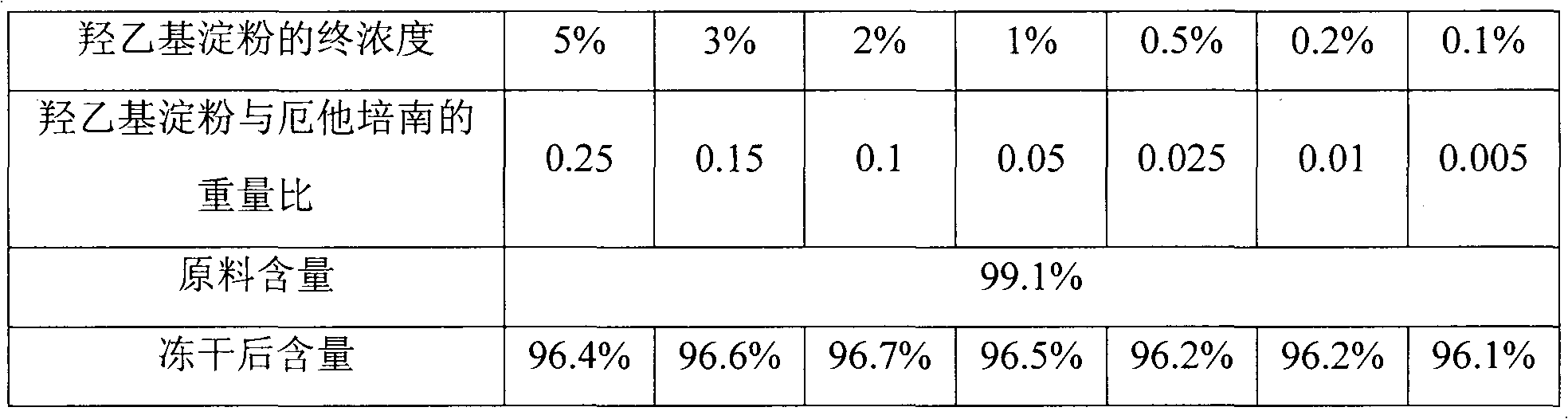
[0021] Weigh 5.0 g of hydroxyethyl starch with an average molecular weight of 130,000 (molar substitution level is 0.4), add water to dissolve to 20 ml, and mix well to obtain a hydroxyethyl starch solution with a concentration of 25%. Then it was gradually diluted to concentrations of 15%, 10%, 5%, 2.5%, 1% and 0.5% (w / v), and placed in a refrigerator at 4°C for cooling. Weigh 7.01g of ertapenem raw material, add 4°C water to dissolve to 28ml and mix well, pipette 4ml into 7 vials respectively. Take 1ml of hydroxyethyl starch solution with a concentration of 25%, 15%, 10%, 5%, 2.5%, 1% and 0.5% into a vial, so that the weight ratio of it to ertapenem is 0.25, 0.15, 0.1, 0.05, 0.025, 0.01, and 0.005. After mixing well, adjust the pH to 7.5±0.3 with 2mol / L NaOH respectively. The 7 tubes of composition solutions were subjected to sterile filtration respectively, and the filtrates were collected in sterilized vials, and then all the samples were pre-frozen at -25°C for 4 hours....
Embodiment 3
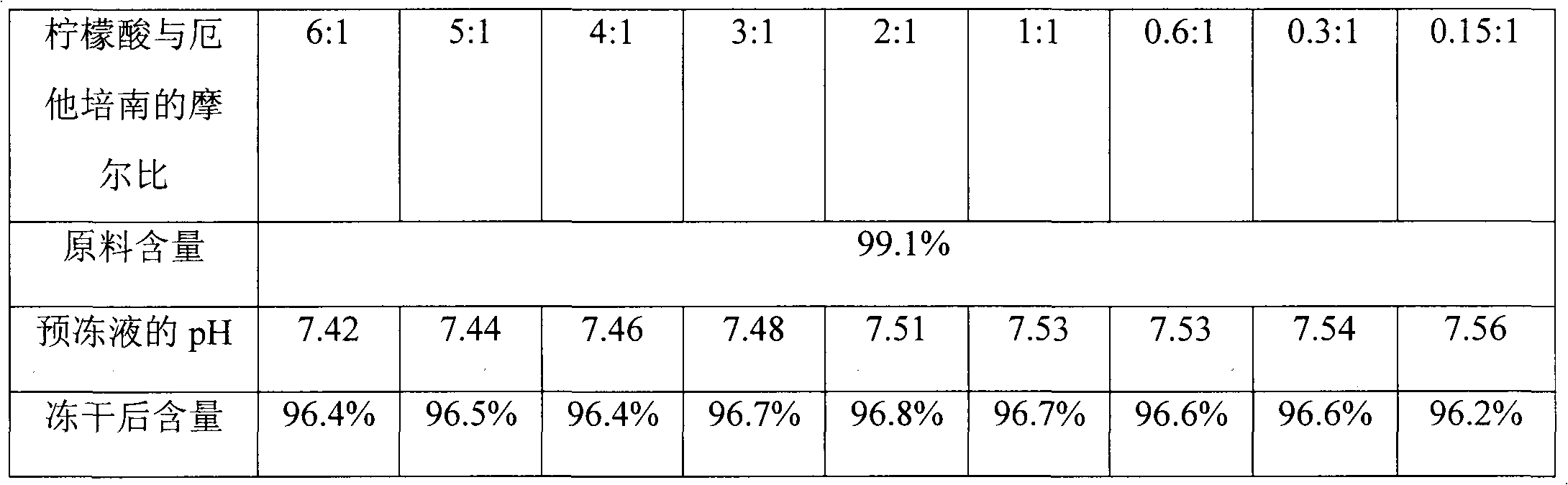
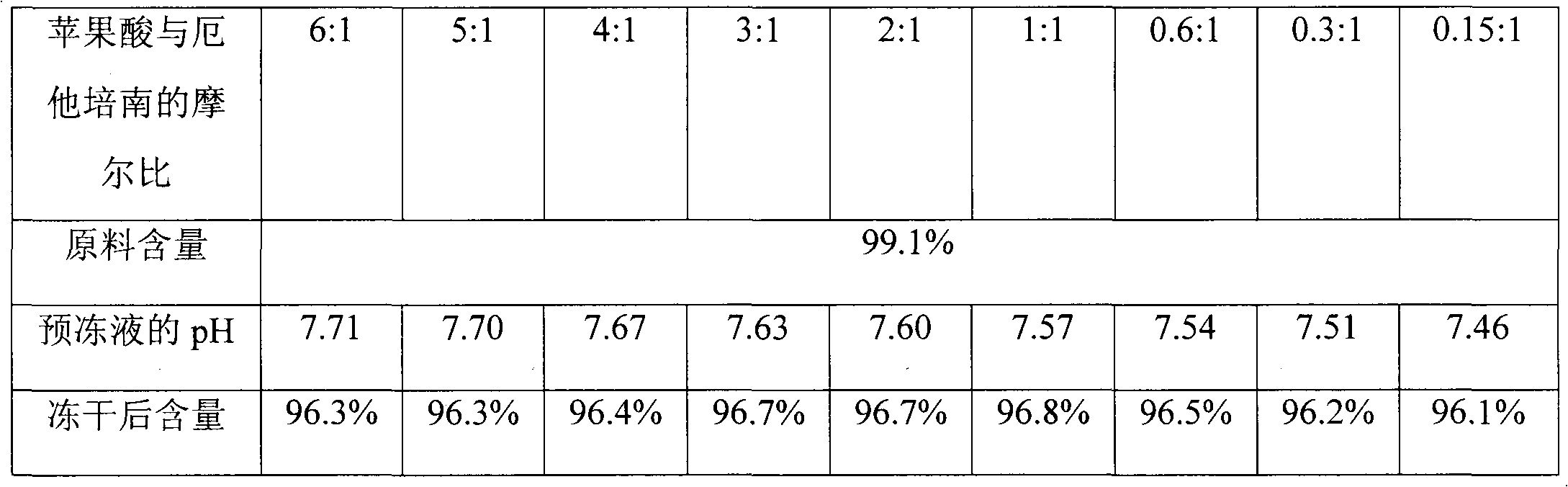
[0026] Weigh 12.10 g of anhydrous citric acid, add 15 ml of water to dissolve it, adjust the pH to 7.40 with 10 mol / L NaOH, add water to make up to a volume of 20 ml, and obtain a 3.15 mol / L citric acid solution. This solution is gradually diluted to a concentration of 2.63mol / L, 2.10mol / L, 1.58mol / L, 1.05mol / L, 0.53mol / L, 0.32mol / L, 0.16mol / L and 0.08mol / L of citric acid The solution was cooled at 4°C for later use.
[0027] Weigh 4.5g of ertapenem raw material, add 20ml of cold water at 4°C to dissolve, adjust the pH to 7.62 with 2mol / L NaOH, add cold water at 4°C to a volume of 27ml, and then divide it into 9 equal portions of 3ml each. Absorb the above pre-cooled 3.15mol / L, 2.63mol / L, 2.10mol / L, 1.58mol / L, 1.05mol / L, 0.53mol / L, 0.32mol / L, 0.16mol / L and 0.08mol / L Add 2ml of each citric acid solution to the ertapenem solution, so that the molar ratios of citric acid and ertapenem are 6, 5, 4, 3, 2, 1, 0.6, 0.3 and 0.15, respectively, and measure after mixing And record the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com