Semi-synthetic method of antineoplastic drug paclitaxel
A paclitaxel and semi-synthetic technology, applied in the five-step reaction of condensation, deprotection, preparation of paclitaxel, protection, selective acetylation, and ring opening, can solve problems such as research without experiments, and achieve fast reaction speed, mild reaction conditions, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
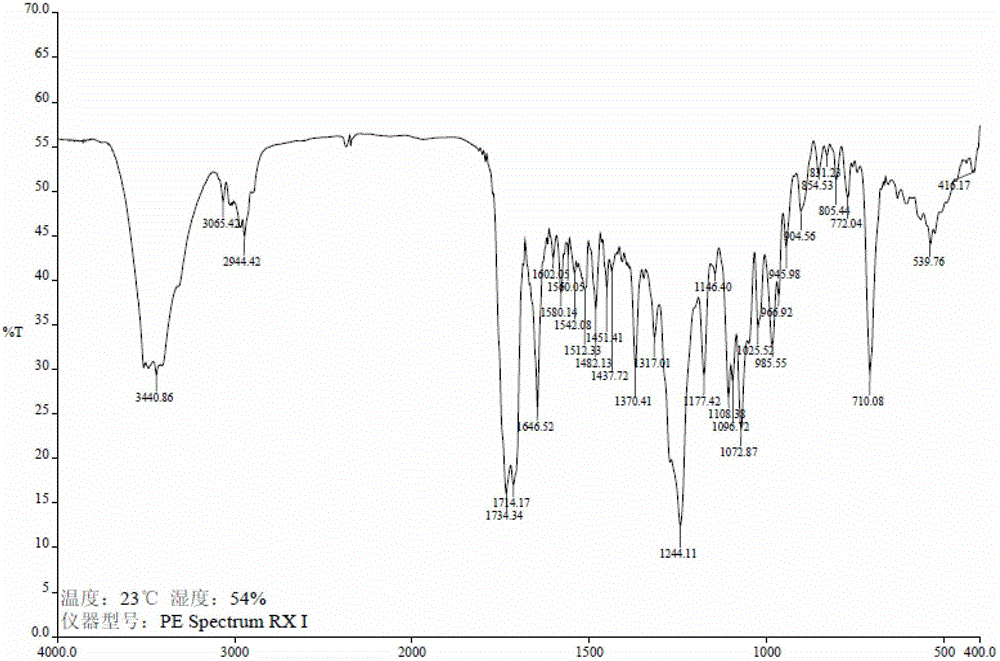
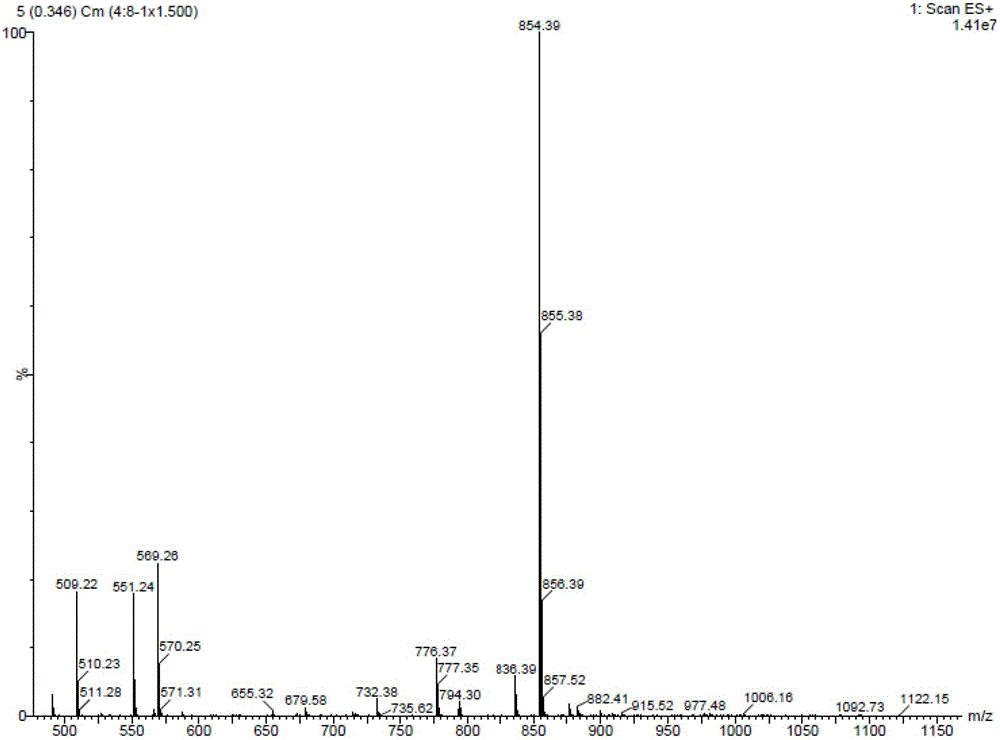
Image
Examples
Embodiment
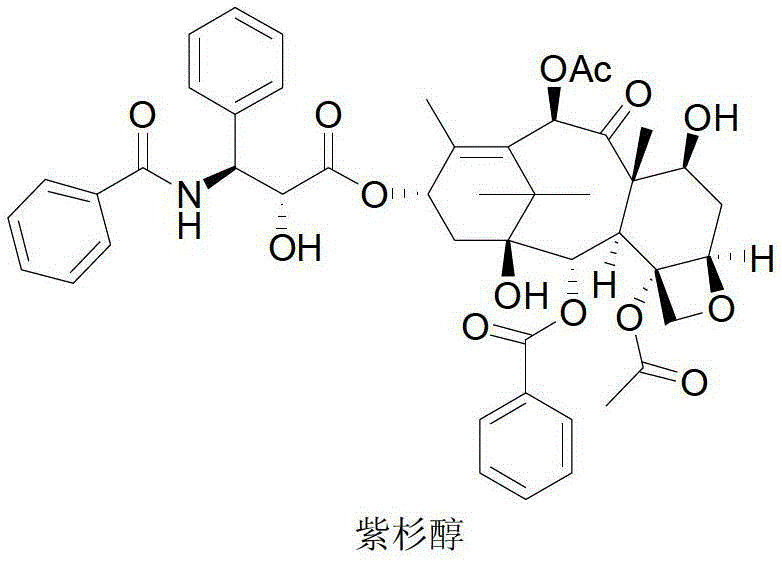
[0043] Paclitaxel semi-synthetic route of the present invention is as follows:
[0044]
[0045] Synthesis of compound 2
[0046] Dissolve 10g (18.4mmol) of 10-deacetylbaccatin III in 1.5L tetrahydrofuran, blow nitrogen gas, stir to dissolve, add 0.684g (1.84mmol) cerium chloride heptahydrate, and cool the reaction solution to 0-5°C. Add 2.81g (27.5mmol) of acetic anhydride dropwise, keep the reaction for 5 hours after dropping, add 1000ml each of ethyl acetate and saturated sodium bicarbonate solution, leave to separate after stirring, and wash the organic layer with saturated brine (1000ml×3) . It was dried over anhydrous sodium sulfate, filtered, and the filtrate was precipitated to obtain 10.9 g of compound 2 (ie, baccatin III), with a yield of 100%.
[0047] Synthesis of Compound 3
[0048] Dissolve 210g (17mmol) of the compound in 100ml of dichloromethane, blow nitrogen, stir to dissolve, add 20ml of anhydrous pyridine, drop the temperature to 0-5°C, and add 5.4g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com