Macromolecule hindered phenol antioxidant, preparation method of macromolecule hindered phenol antioxidant, and application of macromolecule hindered phenol antioxidant
A technology of hindered phenols and antioxidants, applied in the field of antioxidants, can solve the problems of large amount of addition, low antioxidant components, and further improvement of antioxidant performance, and achieve easy process control, large molecular weight, and convenient industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Synthesis of sulfur-containing polyhydroxy polybutadiene: add 10.00 g of hydroxyl-terminated polybutadiene (Kray Willy (Guangzhou) Chemical Co., Ltd., Brand LBH2000, the number average molecular weight is 2.00×10 3, hydroxyl value 0.91mmol / g) and 5.00g tetrahydrofuran, the temperature was raised to 60°C, and magnetic stirring was performed to completely dissolve the hydroxyl-terminated polybutadiene (HTPB). Dissolve 0.200g of azobisisobutyronitrile and 2.00g of 2-mercaptoethanol in 10.00g of tetrahydrofuran, and add it dropwise to the above reactant within 30min, heat and react at 60°C for 3 hours, then transfer the reaction solution to a separate In the liquid funnel, after cooling to 25°C, add excess ice methanol to the separating funnel and oscillate, precipitate and separate into layers to obtain the crude product, then undergo dissolution-precipitation-separation for 5 times, and vacuum-dry at 60°C 12h to obtain sulfur-containing polyhydroxy polybutadiene. Th...
Embodiment 2
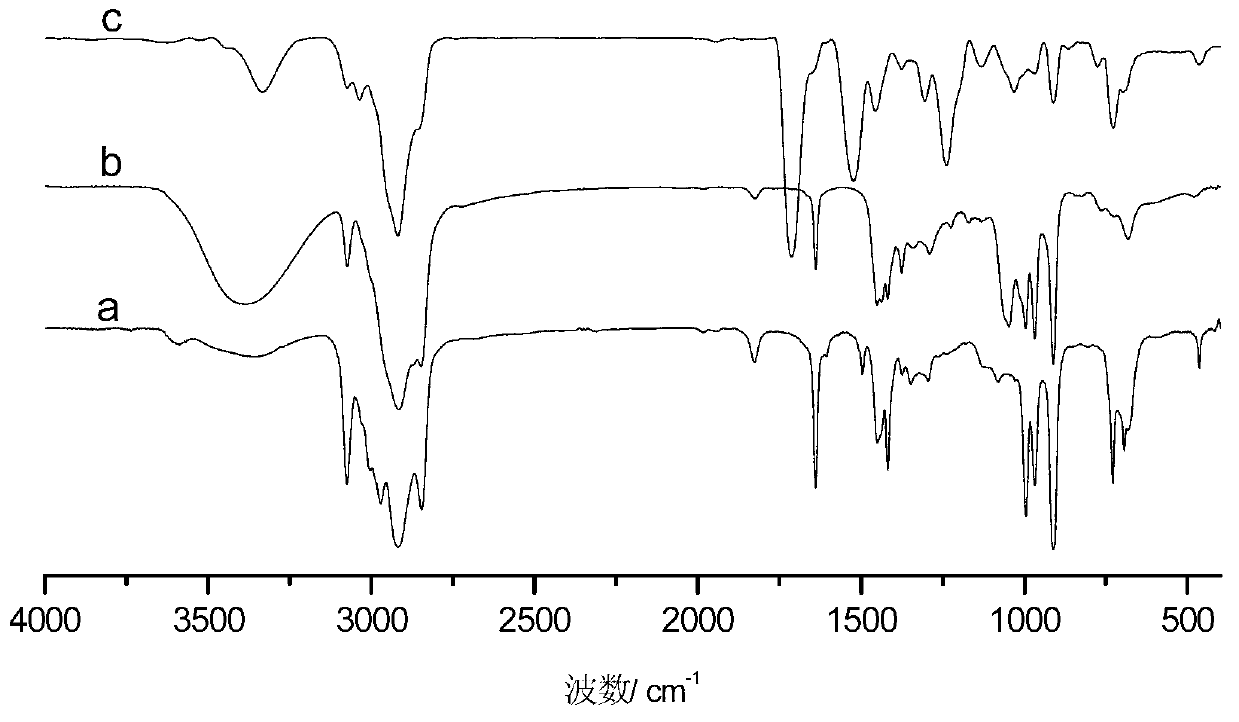
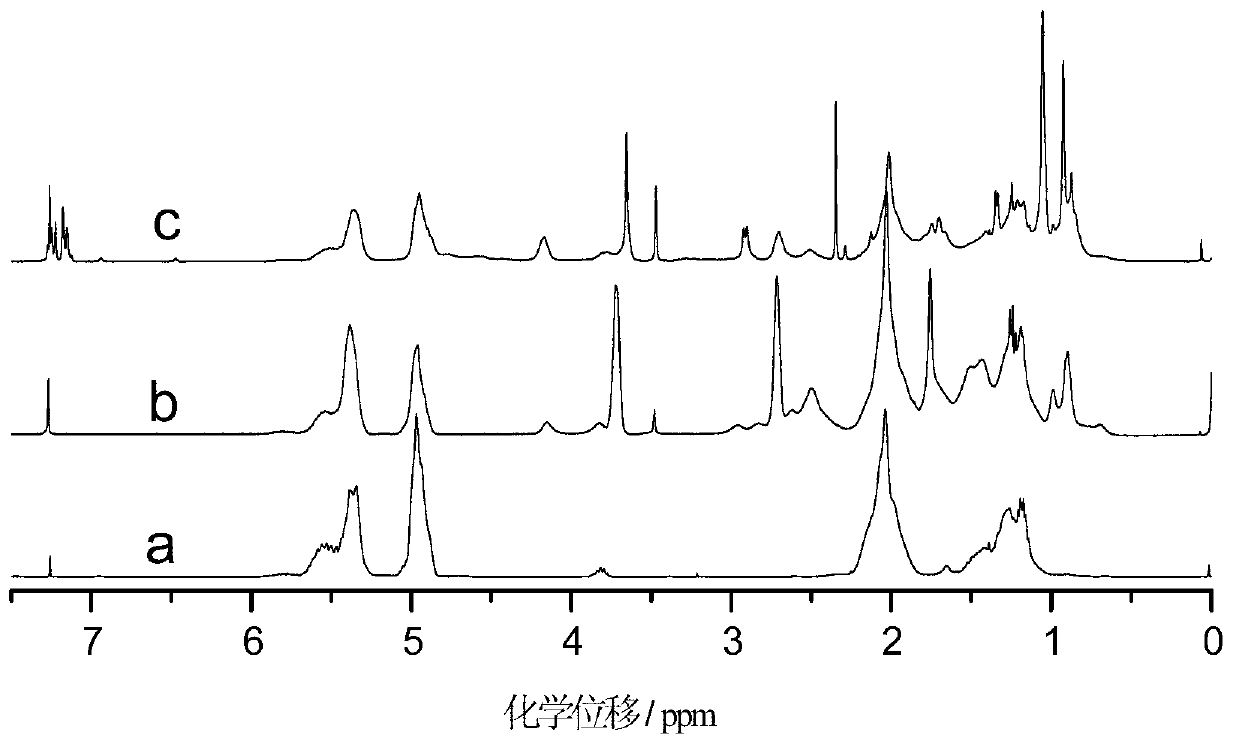
[0036] The difference between this example and Example 1 is that the amount of 2-mercaptoethanol in step (1) is changed to 5.00g, the amount of azobisisobutyronitrile is changed to 0.300g, the reaction time is 5h, and the constant temperature reaction temperature is 70°C . The hydroxyl value of the synthesized sulfur-containing polyhydroxy polybutadiene is 7.24mmol / g. In step (2), the mol ratio of isophorone diisocyanate and 2,2'-thiobis(4-methyl-6-tert-butylphenol) is changed into 1: 1.5, i.e. the ratio of isophorone diisocyanate The consumption is changed to 16.10g, and the consumption of 2,2'-thiobis(4-methyl-6-tert-butylphenol) is changed to 38.88g. by FT-IR and 1 Analysis such as H-NMR can show that the macromolecule hindered phenolic antioxidant has been successfully prepared.
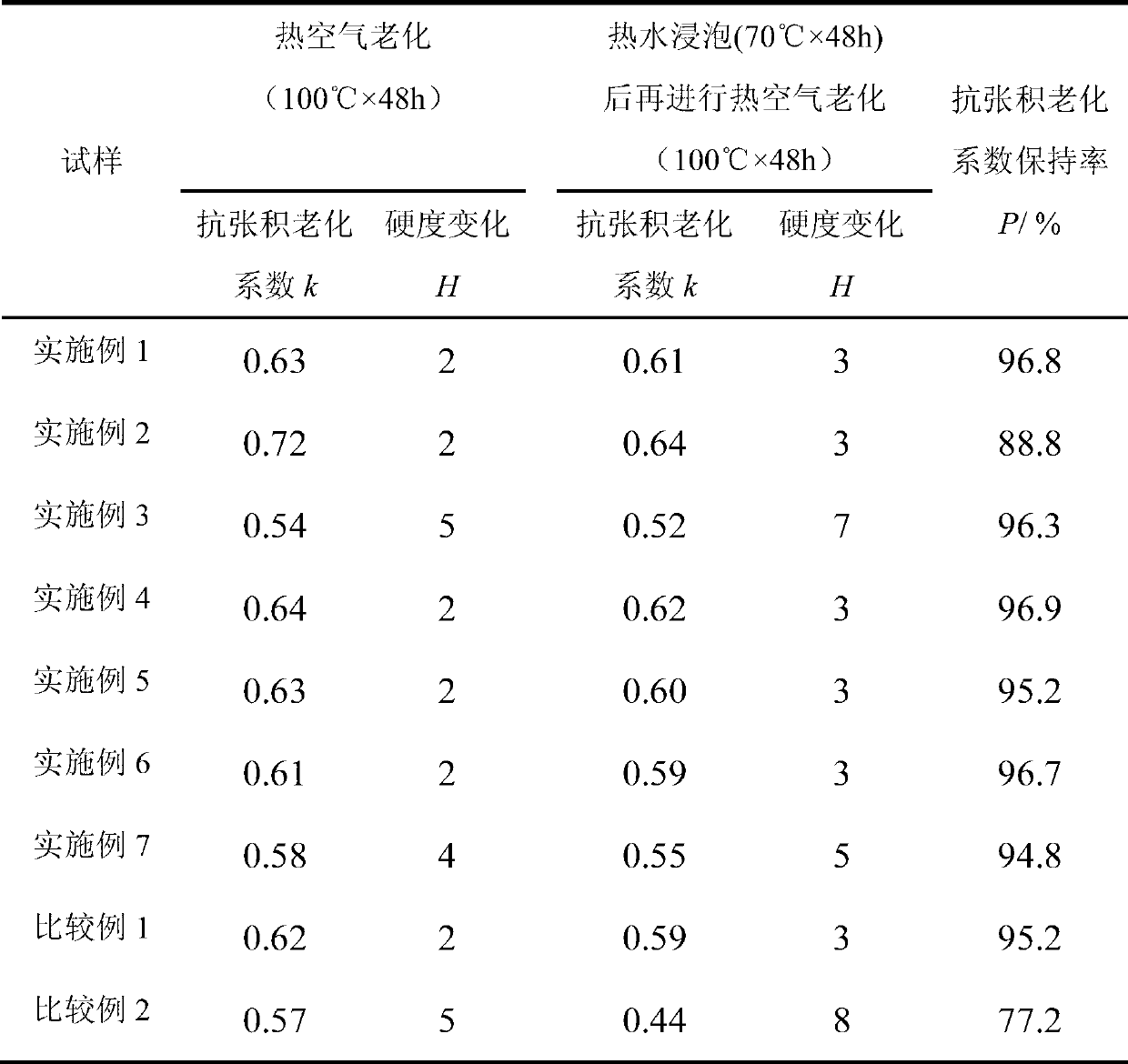
[0037] The thermo-oxidative aging resistance and extraction resistance of the vulcanizate are shown in Table 1. It can be seen from Table 1 that the k value of natural rubber vulcanizate is 0...
Embodiment 3
[0039] This example differs from Example 1 in that the amount of 2-mercaptoethanol used in step (1) was changed to 1.00 g, azobisisobutyronitrile was changed to 0.050 g, the reaction time was 2 hours, and the constant temperature reaction temperature was 50°C. The hydroxyl value of the synthesized sulfur-containing polyhydroxy polybutadiene is 1.32mmol / g. Correspondingly, in step (2), the consumption of isophorone diisocyanate is changed to 2.93g, and the consumption of 2,2'-thiobis(4-methyl-6-tert-butylphenol) is changed to 9.45g. by FT-IR and 1 Analysis such as H-NMR can show that the macromolecule hindered phenolic antioxidant has been successfully prepared.
[0040] The thermo-oxidative aging resistance and extraction resistance of the vulcanizate are shown in Table 1. It can be seen from Table 1 that the k value of natural rubber vulcanizate after 48 hours of thermal oxygen accelerated aging at 100°C is 0.54; after soaking in 70°C water for 48 hours, and then 100°C ther...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com