Macromolecule hindered phenol antioxidant, preparation method of macromolecule hindered phenol antioxidant, and application of macromolecule hindered phenol antioxidant
A technology of hindered phenols and antioxidants, applied in the field of antioxidants, can solve the problems of low antioxidant components, further improvement of antioxidant performance, large addition amount, etc., to achieve large molecular weight, facilitate industrial production, and easy process control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Synthesis of sulfur-containing polyhydroxy polybutadiene: Add 10.00 g of hydroxyl-terminated polybutadiene (Craigville (Guangzhou) Chemical Co., Ltd., Brand LBH2000, the number average molecular weight is 2.00×10 3, the hydroxyl value is 0.91mmol / g) and 5.00g tetrahydrofuran, the temperature is raised to 60°C, and magnetic stirring is used to completely dissolve the hydroxyl-terminated polybutadiene (HTPB). Dissolve 0.200g of azobisisobutyronitrile and 2.00g of 2-mercaptoethanol in 10.00g of tetrahydrofuran, and add them dropwise to the above reactants within 30min, heat and react at 60°C for 3 hours, then transfer the reaction solution to the separatory In the funnel, after cooling to 25°C, add excess ice methanol to the separatory funnel and shake, precipitate and separate into layers to obtain the crude product, then undergo dissolution-precipitation-separation for 5 times, and vacuum-dry at 60°C for 12h Sulfur-containing polyhydroxy polybutadiene is obtained. ...
Embodiment 2
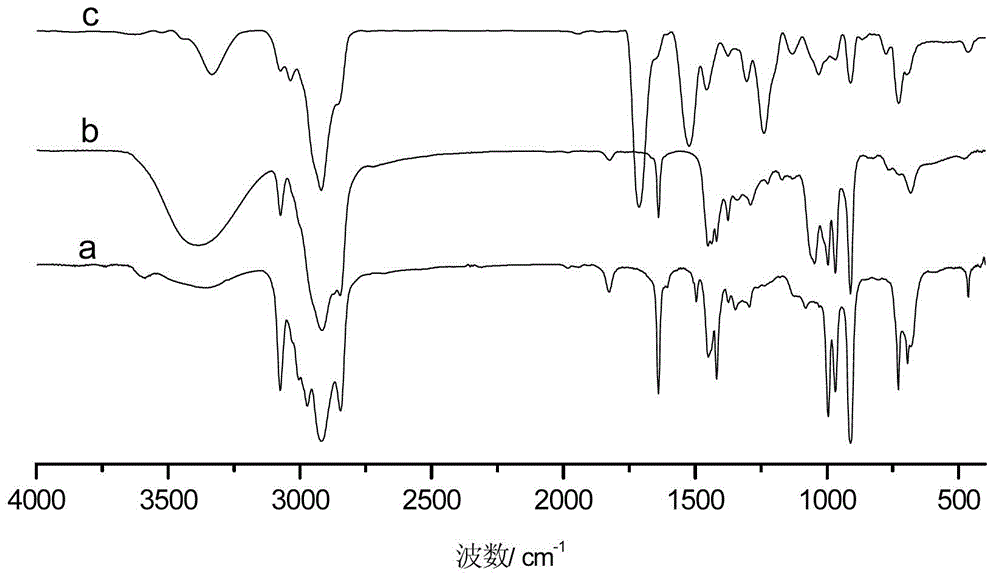
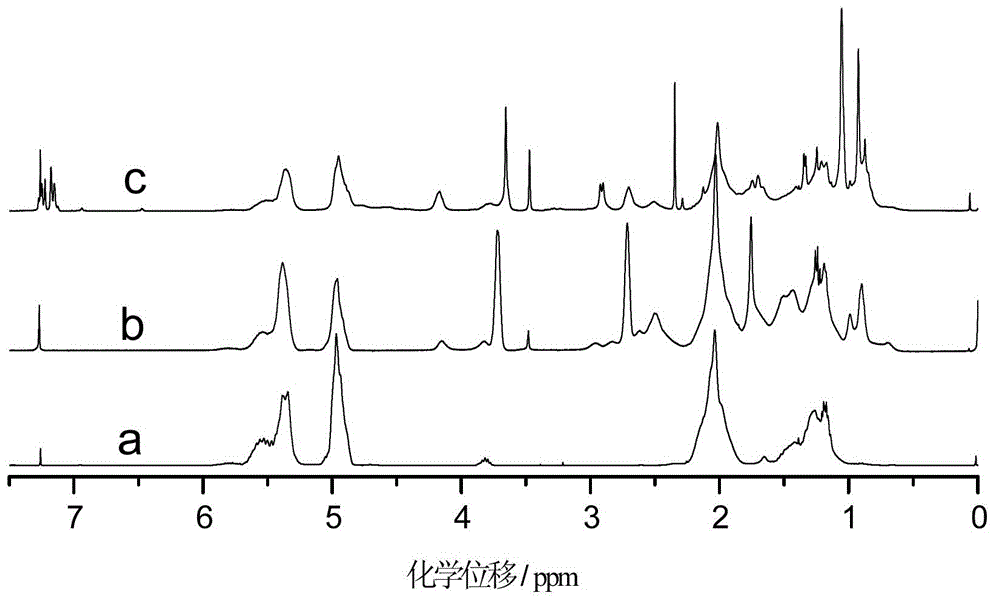
[0036] The difference between this example and Example 1 is that the amount of 2-mercaptoethanol in step (1) is changed to 5.00g, the amount of azobisisobutyronitrile is changed to 0.300g, the reaction time is 5h, and the constant temperature reaction temperature is 70°C . The hydroxyl value of the synthesized sulfur-containing polyhydroxy polybutadiene is 7.24mmol / g. In step (2), the molar ratio of isophorone diisocyanate to 2,2′-thiobis(4-methyl-6-tert-butylphenol) is changed to 1:1.5, that is, the ratio of isophorone diisocyanate The dosage was changed to 16.10g, and the dosage of 2,2′-thiobis(4-methyl-6-tert-butylphenol) was changed to 38.88g. by FT-IR and 1 Analysis such as H-NMR can show that the macromolecule hindered phenolic antioxidant has been successfully prepared.
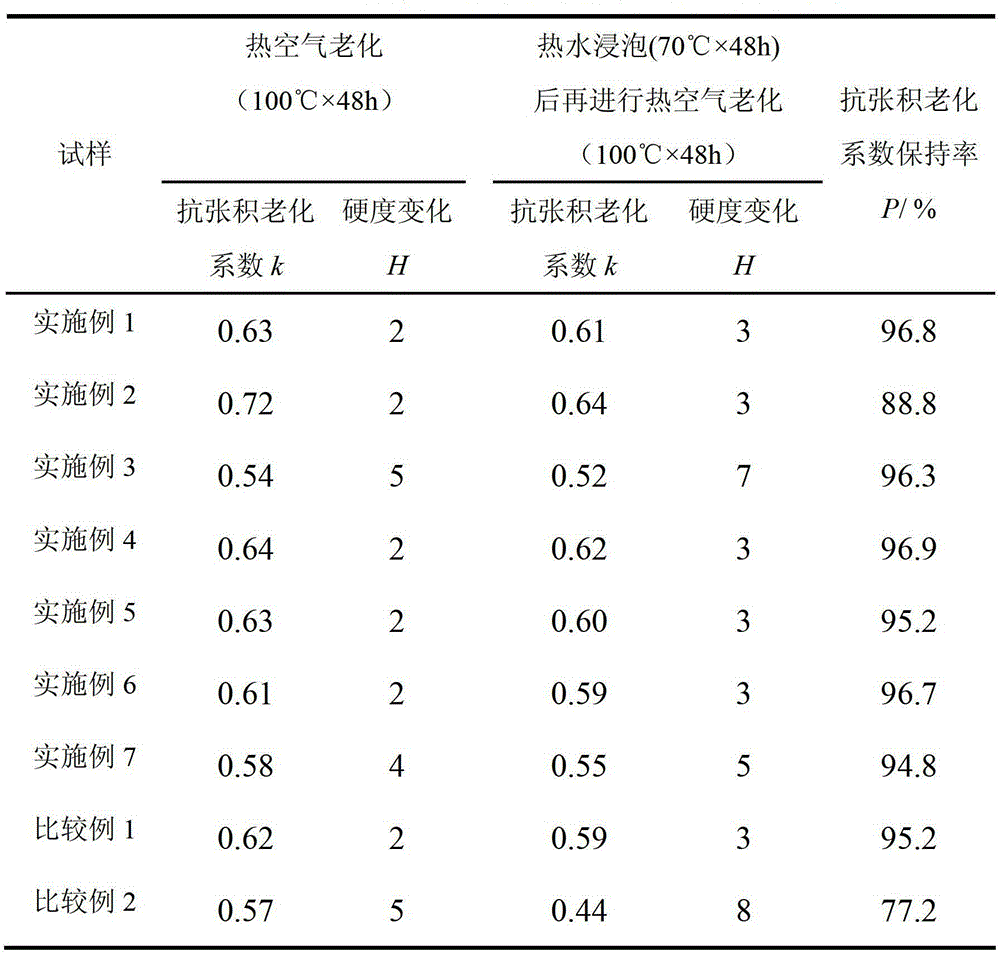
[0037] The thermo-oxidative aging resistance and extraction resistance of the vulcanizate are shown in Table 1. It can be seen from Table 1 that the k value of natural rubber vulcanizate is 0.72 af...
Embodiment 3
[0039] The difference between this example and Example 1 is that the dosage of 2-mercaptoethanol in step (1) was changed to 1.00 g, the amount of azobisisobutyronitrile was changed to 0.050 g, the reaction time was 2 hours, and the constant temperature reaction temperature was 50°C. The hydroxyl value of the synthesized sulfur-containing polyhydroxy polybutadiene is 1.32mmol / g. Correspondingly, in step (2), the amount of isophorone diisocyanate was changed to 2.93 g, and the amount of 2,2′-thiobis(4-methyl-6-tert-butylphenol) was changed to 9.45 g. by FT-IR and 1 Analysis such as H-NMR can show that the macromolecule hindered phenolic antioxidant has been successfully prepared.
[0040] The thermo-oxidative aging resistance and extraction resistance of the vulcanizate are shown in Table 1. It can be seen from Table 1 that the k value of natural rubber vulcanizate after 48 hours of thermal oxygen accelerated aging at 100°C is 0.54; after soaking in 70°C water for 48 hours, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com