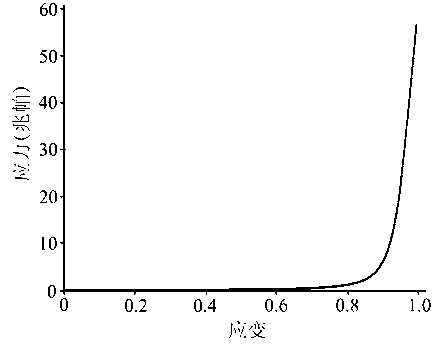
High-strength hydrogel
A hydrogel, high-strength technology, applied in prosthesis, medical science, etc., can solve problems such as impact on application and low mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Dissolve 0.5 g of sodium hyaluronate (HA) in 100 mL of phosphate buffer solution (PBS, pH 7.4), add 50 mL of N,N-dimethylformamide, and then sequentially add 2.52 g of triethylamine and 3.54 g glycidyl methacrylate, stirred at room temperature for 5 days. Concentrate, dialyze, and lyophilize to obtain double-bond functionalized hyaluronic acid (HAGMA); dissolve 2 g of PEG with a number average molecular weight of 20,000 g / mol into a 100-ml round-bottomed flask filled with 50 ml of toluene at 135 °C Under reflux for 4 hours, a small amount of water was removed by azeotropic distillation. After the toluene solution is cooled to room temperature, add 0.13 g of sodium carbonate, stir in an ice bath for 30 minutes, then slowly drop 0.1 mL of acryloyl chloride dissolved in 10 ml of tetrahydrofuran into the flask, and react in an ice bath for 30 minutes , react overnight at 45°C. After the reaction was completed, it was filtered with suction, concentrated and repr...
Embodiment 2
[0021] Example 2: Dissolve 0.5 g of sodium polyglutamate (PGA) in 20 mL of phosphate buffer solution (PBS, pH 7.4), then add 2.52 g of dimethylaminopyridine and 3.54 g of glycidyl methacrylate in sequence, and stir at room temperature 5 days. Concentrate, dialyze, and lyophilize to obtain double-bond functionalized sodium polyglutamate (PGAGMA); dissolve 2 g of PEG with a number average molecular weight of 20,000 g / mol into a 100 mL round-bottomed flask containing 50 mL of toluene , 135 degrees Celsius under reflux for 4 hours, azeotropic distillation to remove traces of water. After the toluene solution is cooled to room temperature, add 0.13 g of sodium carbonate, stir in an ice bath for 30 minutes, then slowly drop 0.1 mL of acryloyl chloride dissolved in 10 ml of tetrahydrofuran into the flask, and react in an ice bath for 30 minutes , react overnight at 45°C. After the reaction was completed, it was filtered with suction, concentrated and reprecipitated in excess ether,...
Embodiment 3
[0022] Example 3: Dissolve 0.5 g of sodium chondroitin sulfate (CS) in 50 mL of phosphate buffer solution (PBS, pH 7.4), add 50 mL of N,N-dimethylformamide, and then add 2.52 g of triethylamine in sequence and 3.54 g glycidyl methacrylate, stirred at room temperature for 5 days. Concentrate, dialyze, and lyophilize to obtain double-bond functionalized chondroitin sulfate sodium (CSGMA); dissolve 2 g of PEG with a number-average molecular weight of 20,000 g / mol into a 100-mL round-bottomed flask containing 50 mL of toluene, Reflux at 135 degrees Celsius for 4 hours, and remove a small amount of water by azeotropic distillation. After the toluene solution is cooled to room temperature, add 0.13 g of sodium carbonate, stir in an ice bath for 30 minutes, then slowly drop 0.1 mL of acryloyl chloride dissolved in 10 ml of tetrahydrofuran into the flask, and react in an ice bath for 30 minutes , react overnight at 45°C. After the reaction was completed, it was filtered with suction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com