Docetaxel semi-synthesis method
A docetaxel and semi-synthetic technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of harsh reaction conditions and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
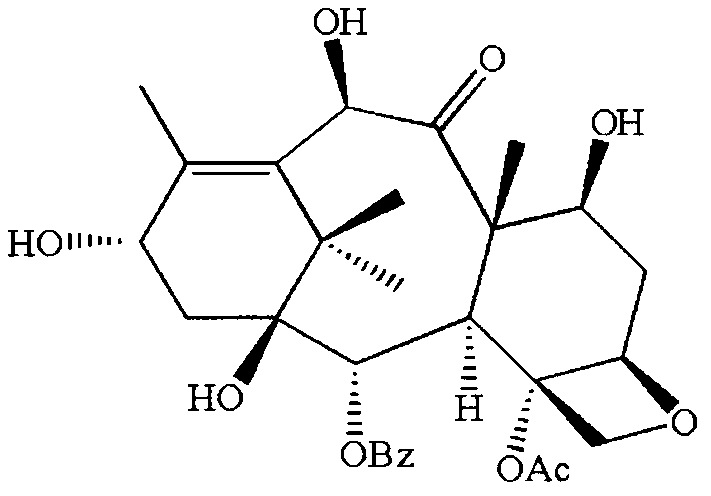
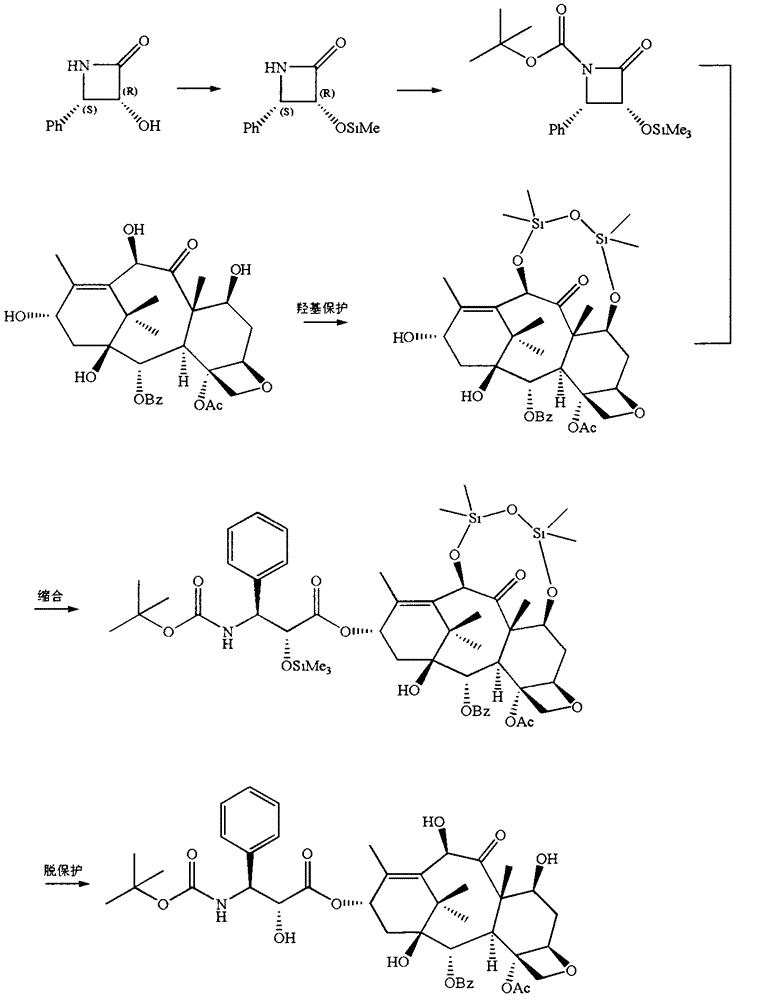
[0026] Embodiment 1 (semi-synthetic reaction formula sees figure 2 )
[0027] (1) Hydroxyl protection of side chain precursor
[0028] Dissolve 3.4 g of optically active side chain precursors in 30 ml of tetrahydrofuran (THF), and then slowly add 5.8 g of triethylamine, 76 mg of 4-dimethylaminopyridine (DMAP) and 2.4 g of β-(trimethylsilyl ) ethoxymethyl chloride, and the mixture was stirred at 0°C for 30 minutes. During the spot plate, when it is completely converted into a less polar product, add 30mL ethyl acetate to dilute, then wash with 15ml saturated aqueous sodium bicarbonate solution and 15ml brine, and dry with 5g sodium sulfate, filter, and concentrate the filtrate. Recrystallized from alkane to obtain a white powder. Vacuum-filtered on a Buchner funnel, and vacuum-dried at room temperature to a constant weight of 3.45 g, yield 72%.
[0029] (2) Side chain C 3’ tert-butoxycarbonyl
[0030] Take 0.95 g of the product with optical activity after the previous st...
Embodiment 2
[0036] (1) Hydroxyl protection of side chain precursor
[0037] Dissolve 3.4 g of optically active side chain precursors in 30 ml of tetrahydrofuran, and then slowly add 5.8 g of triethylamine, 76 mg of 4-dimethylaminopyridine (DMAP) and 2.4 g of β-(trimethylsilyl)ethoxy methyl chloride, and the mixture was stirred at 0°C for 30 minutes. During the spot plate, when it is completely converted into a less polar product, add 30mL ethyl acetate to dilute, then wash with 15ml saturated aqueous sodium bicarbonate solution and 15ml brine, and dry with 5g sodium sulfate, filter, and concentrate the filtrate. Recrystallized from alkane to obtain a white powder. Vacuum-filtered on a Buchner funnel, and vacuum-dried at room temperature to a constant weight of 3.45 g, yield 72%.
[0038] (2) Side chain C 3’ tert-butoxycarbonyl
[0039] Take 0.95 g of the product with optical activity after the previous step of purification and dissolve it in 10 ml of tetrahydrofuran, then slowly add 1...
Embodiment 3
[0045] (1) Hydroxyl protection of side chain precursor
[0046] Dissolve 3.4 g of optically active side chain precursors in 30 ml of tetrahydrofuran, and then slowly add 5.8 g of triethylamine, 76 mg of 4-dimethylaminopyridine (DMAP) and 2.4 g of β-(trimethylsilyl)ethoxy methyl chloride, and the mixture was stirred at 0°C for 30 minutes. During the spot plate, when it is completely converted into a less polar product, add 30mL ethyl acetate to dilute, then wash with 15ml saturated aqueous sodium bicarbonate solution and 15ml brine, and dry with 5g sodium sulfate, filter, and concentrate the filtrate. Recrystallized from alkane to obtain a white powder. Vacuum-filtered on a Buchner funnel, and vacuum-dried at room temperature to a constant weight of 3.45 g, yield 72%.
[0047] (2) Side chain C 3’ tert-butoxycarbonyl
[0048] Take 0.95 g of the product with optical activity after the previous step of purification and dissolve it in 10 ml of tetrahydrofuran, then slowly add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com