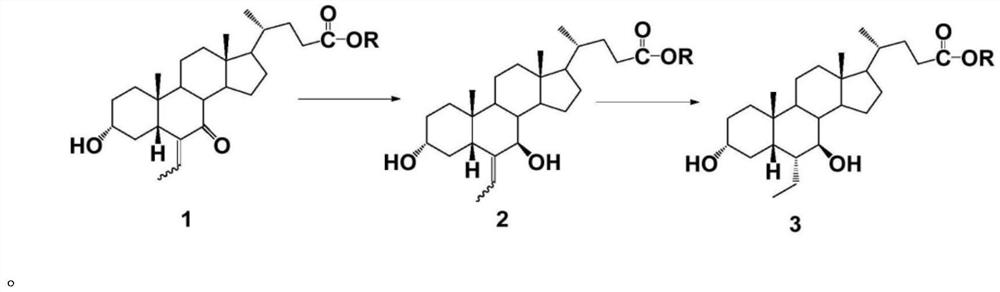
A kind of preparation method of 6-alpha-ethyl-7-keto gallstone acid
A ketocholic acid and ethyl technology, which is applied in the fields of synthetic chemistry and biomedicine, can solve the problems of complex product system, excessive side reactions, low actual yield and the like, and achieves simplified process, mild reaction conditions and high yield. Excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
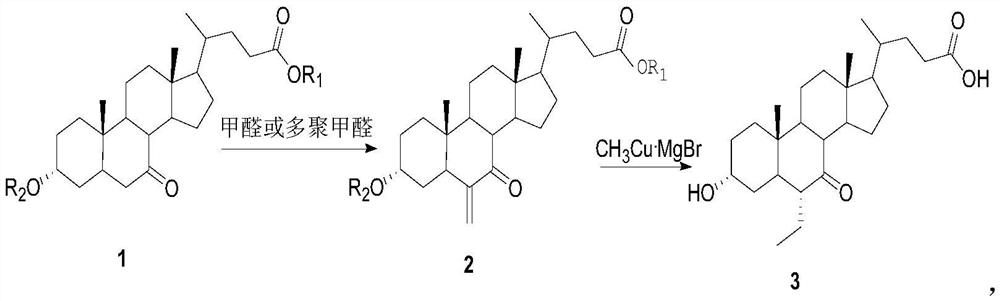
[0035] Add 390g (about 1mol) of methyl 7-ketocholelithate (compound 1) into a 5L reaction flask, add 150g (5mol) of paraformaldehyde, 2.5L of toluene, and 276g (about 2mol) of potassium carbonate, at 60°C The reaction was vigorously stirred, followed by TLC spot plate (petroleum ether: ethyl acetate = 1:1, color developed by ammonium phosphomolybdate baked plate), the raw materials basically disappeared, and the reaction was stopped. After the reaction system was cooled, it was filtered. After the toluene solution was filtered out, it was evaporated to dryness to obtain methyl 6-methylene-7-ketocholelithate (compound 2). Methyl 6-methylene-7-ketocholelithate was purified by silica gel column chromatography.
[0036]Dissolve 2 mol of the methyl Grignard reagent MeMgBr in 2 L of anhydrous tetrahydrofuran, cool down to -20°C, add 217 g (2.2 mol) of cuprous chloride under nitrogen protection, stir for 10 minutes to half an hour, and a yellow solid suspension appears. This is the ...
Embodiment 2
[0038] Add 390g (about 1mol) of methyl 7-ketocholelithate to a 5L reaction flask, add 2.5L of DMF, 150g (5mol) of paraformaldehyde, and 357g (1.1mol) of cesium carbonate, and stir vigorously at 60°C. TLC plate tracking (petroleum ether: ethyl acetate = 1:1, ammonium phosphomolybdate baked plate for color development), the raw materials had disappeared, and the reaction was stopped.
[0039] The excess DMF solvent was distilled off under reduced pressure, and the residual material was extracted twice with diethyl ether (1.2 L). The diethyl ether solution was washed with water and dried over anhydrous sodium sulfate. After passing the drying, lower the temperature to -20°C, add the pre-cooled ether solution (11mol) of dimethyl copper lithium reagent at this temperature, raise the temperature to 0°C naturally, continue to react at about 0°C for 1 hour, add dropwise cold 2L of 5% dilute hydrochloric acid aqueous solution was used to quench the reaction, stirred for 2 hours, and th...
Embodiment 3
[0041] 390g (about 1mol) of ethyl 7-ketocholelithate (compound 1) was added in a 5L reaction flask, 5mol of anhydrous formaldehyde gas was fed into it, 2.5L of tetrahydrofuran was added, and 207g of potassium carbonate (about 1.5mol) was added at 20 The reaction was vigorously stirred at ℃, followed by TLC plate tracking (petroleum ether: ethyl acetate = 1:1, ammonium phosphomolybdate baked plate for color development), the raw materials basically disappeared, and the reaction was stopped. After the reaction system was cooled, it was filtered and evaporated to dryness to obtain ethyl 6-methylene-7-ketocholelithate (compound 2). Ethyl 6-methylene-7-ketocholelithate was purified by silica gel column chromatography.
[0042] Dissolve compound 2 in 800ml tetrahydrofuran, pre-cool to -30°C, then add it into dimethyl copper lithium reagent, stir for 0.5 hours, then slowly raise the temperature to 10°C, continue to stir for 1 hour, add cold 3% Quench the reaction with 2L of dilute h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com