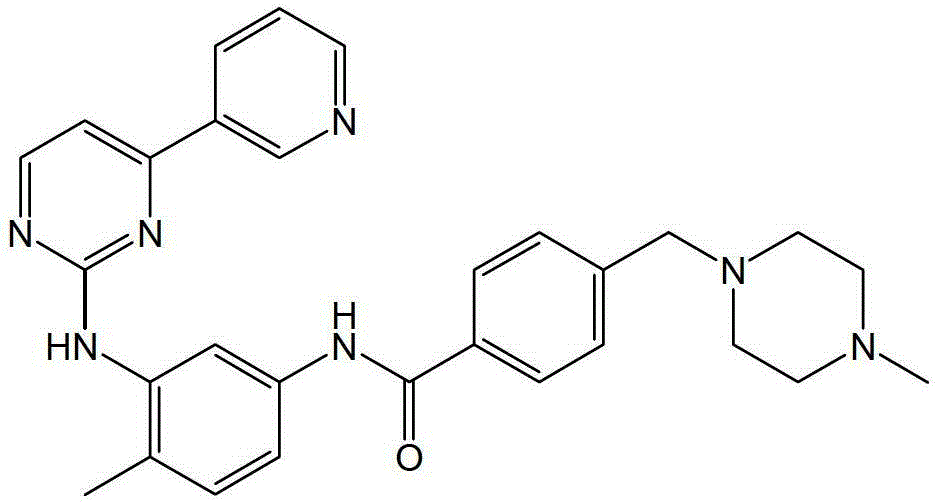
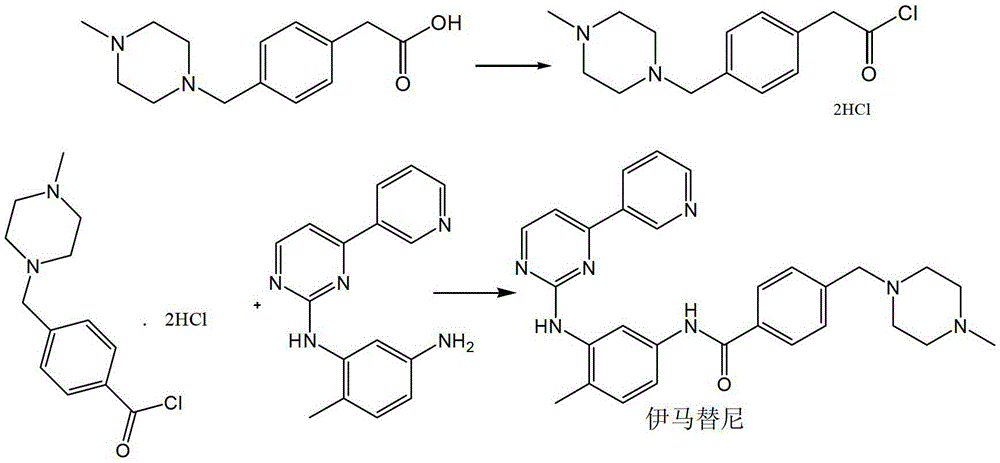
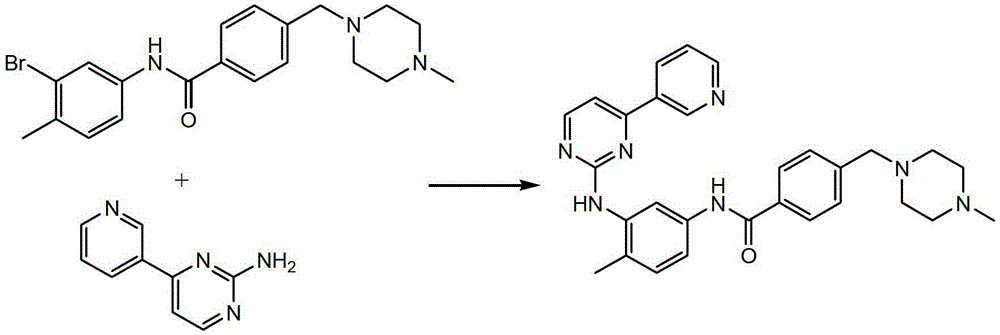
Preparation method of imatinib
A technology of imaamine and halomethylbenzoic acid, which is applied in the field of imatinib preparation, can solve the problems of large environmental pollution, cumbersome operation, and many impurities, and achieve high product yield, simple operation, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In a 100ml three-necked flask equipped with a stirring and thermometer, add 50ml of dichloromethane and 3.4g of p-chloromethylbenzoic acid, stir at room temperature, add 1.6g of sodium hydroxide and stir, and add 2.4g of pivaloyl chloride dropwise under control of 30°C. React for 0.5 hours. After the reaction is complete, add a DMF solution of imamamine (2.77g / 50ml), keep stirring for 3 hours, filter, rinse with 10ml of dichloromethane, rinse once with 10ml of purified water, and dry under reduced pressure at 40-60°C to obtain Intermediate 2 is 7.74g, and the yield is 90.00%.
Embodiment 2
[0056] In a 100ml three-necked flask equipped with a stirring and thermometer, add 50ml of dichloromethane and 4.3g of p-bromomethylbenzoic acid, stir at room temperature, add 2.3g of triethylamine until it dissolves, and add dropwise at -30~-25℃ 1.9 g of pivaloyl chloride was reacted for 1.5 hours. After the reaction is completed, add imamamine in DMF (11.1g / 50ml), heat up to 0-25°C and stir for 3 hours, filter, rinse with 10ml of dichloromethane, rinse once with 10ml of purified water, and reduce the temperature at 40-60°C. After vacuum drying, 8.1 g of Intermediate II was obtained with a yield of 94.19%.
Embodiment 3
[0058] In a 100ml three-necked flask equipped with a stirring and thermometer, add 50ml of dichloromethane and 3.4g of p-chloromethylbenzoic acid, stir at room temperature, add 2.3g of triethylamine until it dissolves with stirring, and dropwise add pentapentyl at 0-20°C Acyl chloride 4.8g, reacted for 1 hour. After the reaction is completed, add a DMF solution of imamamine (4.4g / 50ml), keep stirring for 3 hours, filter, rinse with 10ml of dichloromethane, rinse once with 10ml of purified water, and dry under reduced pressure at 40-60°C to obtain Intermediate 2 is 8.2g, and the yield is 95.34%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com