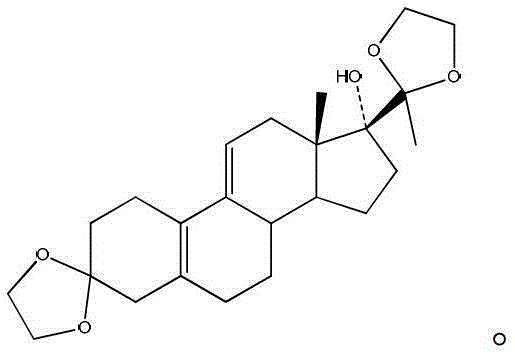
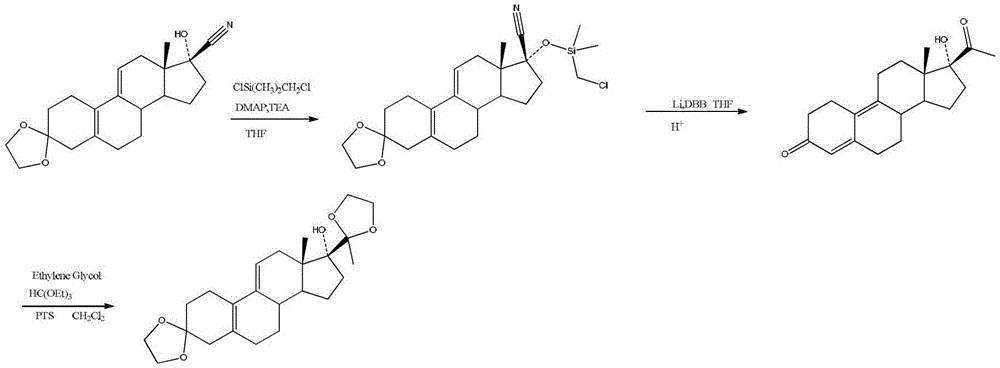
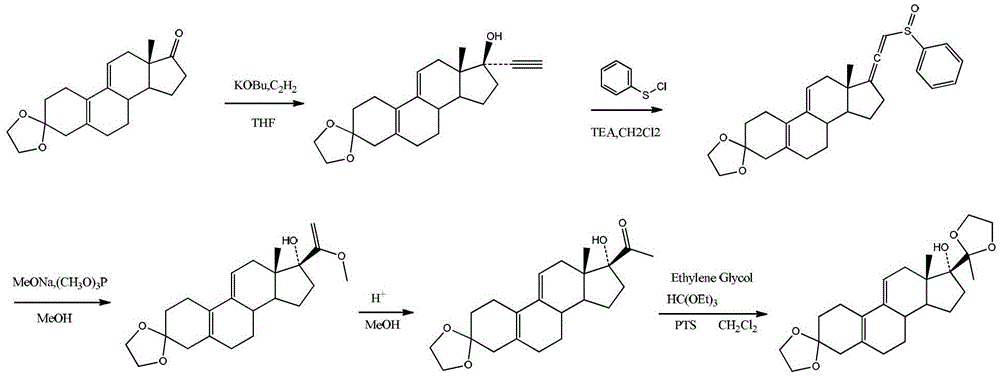
Preparation method of ulipristal acetate key intermediate
A technology of uliplast acetate and intermediates, which is applied in the field of compound preparation, can solve the problems of high waste water post-treatment cost, wide fluctuation range of yield, poor reaction stability, etc., and achieve broad industrial application prospects, stable process and high product quality. Effect of Yield Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the key intermediate of uliplast acetate of the present invention adopts the chemical formula of 3-(ethylenedioxy)-21-(phenyl-sulfinyl)-19-desmethylpregna-5 (10), 9(11), 17(20), 20-tetraene as the starting material, in a reaction tank, use a one-pot method to complete the three-step reaction to obtain the general structure shown in formula I or formula II The compound, which is a structurally similar bisketal compound, is an equivalent intermediate in chemical synthesis;
[0031]
[0032]
[0033] Specifically include the following steps:
[0034] In the first step, 3-(ethylenedioxy)-21-(phenyl-sulfinyl)-19-desmethylpregna-5(10), 9(11), 17(20), 20-tetraene reacts with alcohol;
[0035] In the second step, the reaction solution obtained in the first step is reacted with phosphite to obtain an enol ether compound;
[0036] In the third step, acid is added to the reaction liquid obtained in the second step, and the reaction is stirred at ...
Embodiment 1
[0051] Embodiment 1: the synthesis of compound 1
[0052] Under the protection of nitrogen, add 120ml of methanol and 0.48g of sodium methoxide into the three-necked flask, and stir well; add 20g of 3-(ethylenedioxy)-21-(phenyl-sulfinyl)-19-desmethylpregnan Steroid-5(10), 9(11), 17(20), 20-tetraene, keep warm at 20-25°C and stir for 24 hours, then add 5.5ml trimethyl phosphite, keep warm at 53-57°C and stir for reaction 3 After 1 hour, 2ml of phosphoric acid was added and stirred; after 60 minutes, the reaction was complete; the temperature was lowered to 3°C, and filtered; the filter cake was rinsed with 20ml of methanol; after drying, 12g of compound 1 was obtained.
Embodiment 2
[0053] Embodiment 2: the synthesis of compound 2
[0054] Add 120ml of ethanol and 0.48g of sodium methoxide into a three-necked flask, stir well, add 20g of 3-(ethylenedioxy)-21-(phenyl-sulfinyl)-19-desmethylpregna-5 ( 10), 9(11), 17(20), 20-tetraene, heat up to 38-40°C and stir for reaction; after 16 hours, add 12ml of triethyl phosphite, keep warm at 54-56°C and stir for reaction; after 3 hours , cooled to room temperature; added 1.4ml of trifluoromethanesulfonic acid and stirred; after 55 minutes, cooled to 0°C and filtered; the filter cake was rinsed with 20ml of ethanol; after drying, 12.68g of compound 2 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com