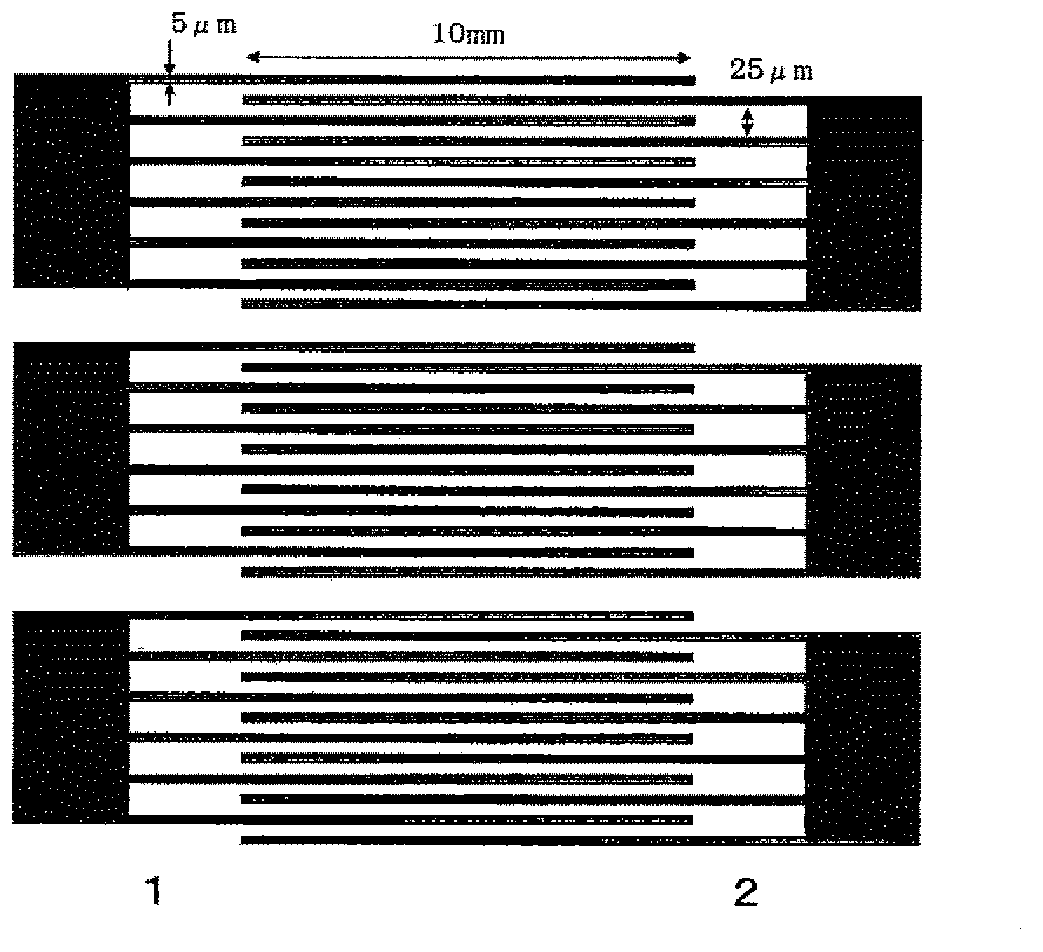
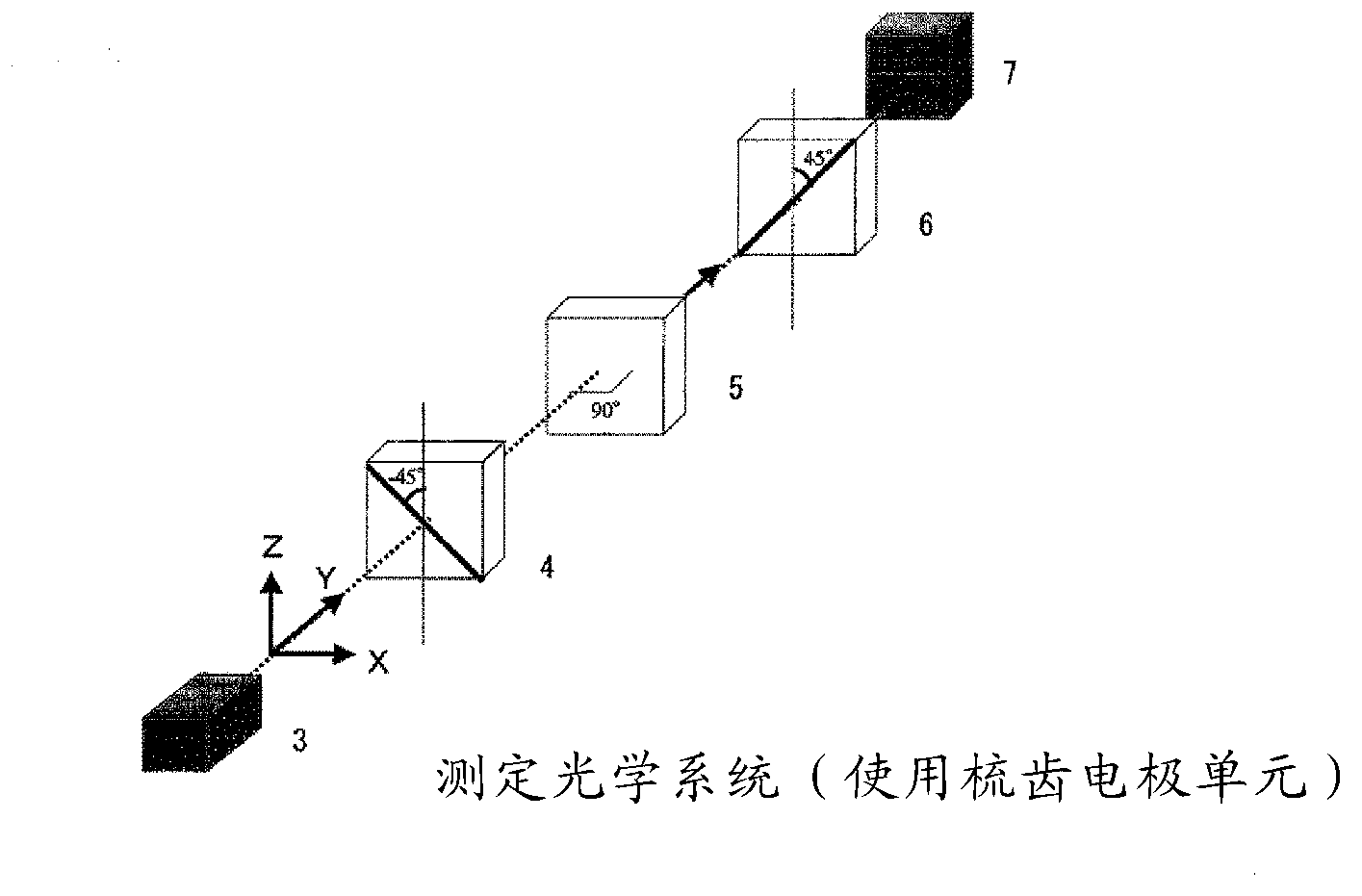
Optically isotropic liquid crystal medium and optical device
An optical isotropic, liquid crystal composition technology, used in optics, liquid crystal materials, nonlinear optics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0251] The liquid crystal composition of the present invention is usually prepared by a known method, for example, a method of dissolving essential components at high temperature.
[0252] 6 Compositions with optically isotropic liquid crystal phases
[0253] 6.1 Composition of Compositions with Optically Isotropic Liquid Crystal Phases
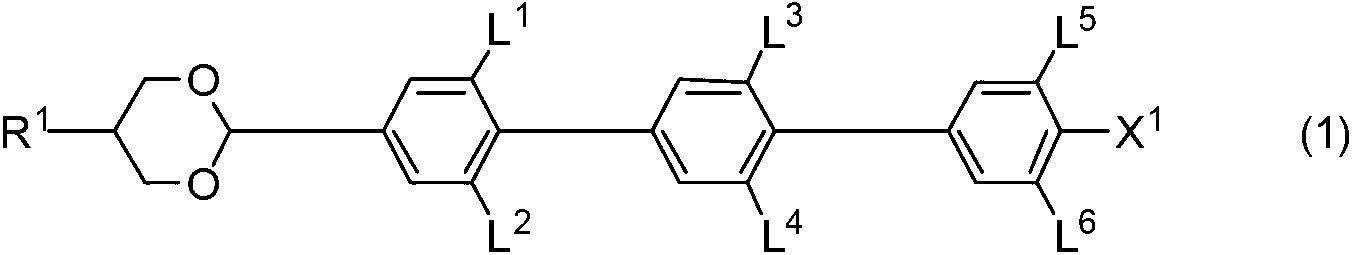
[0254] The sixth aspect of the present invention is a composition comprising an achiral component T and a chiral agent, and is a liquid crystal composition that can be used for an optical device driven by an optically isotropic liquid crystal phase. The achiral component T contains a component A containing compounds represented by formula (1) and formula (2) and formula (3) as addition components. The achiral component T contains, in addition to component A, a compound selected from the group consisting of the fourth component represented by formula (4) and the fifth component represented by formula (5) as needed. The liquid crystal composi...
example
[0357] The compounds obtained are based on the 1 H-NMR ( 1 H-nuclear magnetic resonance, 1 H-NMR) analysis obtained by the nuclear magnetic resonance spectrum, by gas chromatography (gas chromatography, GC) analysis obtained by gas chromatograms, etc., so the analysis method will first be described.
[0358] 1 H-NMR analysis: DRX-500 (manufactured by Bruker BioSpin Co., Ltd.) was used as a measurement device. The measurement is carried out by dissolving the sample produced in Examples etc. in a deuterated solvent such as CDCl3, which is soluble in the sample, at room temperature at 500 MHz, and the accumulation times are 24 times. In addition, in the description of the obtained nuclear magnetic resonance spectrum, s means a singlet, d means a doublet, t means a triplet, q means a quartet, and m means a multiplet. In addition, tetramethylsilane (TMS) was used as the reference substance of the zero point of the chemical shift δ value.
[0359] GC analysis: The measurement d...
Synthetic example 1
[0387] Synthesis of formula (S1-4)
[0388]
[0389] The synthesis process is shown in the figure below.
[0390]
[0391] Synthesis of compound (S1-2)
[0392] Into a reactor under a nitrogen atmosphere, 100 ml of a 1.3 mol / L tetrahydrofuran (THF) solution of isopropylmagnesium chloride and lithium chloride salt was added, and 41.6 g of compound (S1-1) was slowly added thereto at room temperature. After stirring at this temperature for 90 minutes, the reaction liquid was cooled to 0° C., a THF (30 ml) solution of 15.2 g of dimethylformamide was added dropwise, and stirred at room temperature for 12 hours. 2N-hydrochloric acid (200 ml) was dropped into the reaction liquid, 1 L of a mixed solvent of toluene / diethyl ether=1 / 1 (volume ratio) was added thereto, the product was extracted, and the organic layer was washed with water. After drying over magnesium sulfate, the solvent was distilled off under reduced pressure. Using toluene / ethyl acetate=5 / 1 (volu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com