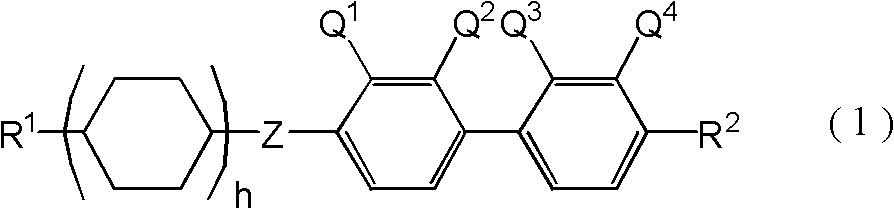
Liquid crystal compound having negative dielectric anisotropy, liquid crystal composition using this and liquid crystal display device
A technology of liquid crystal composition and compound, which is applied in the field of liquid crystal composition and novel liquid crystal compound, to achieve the effects of excellent compatibility, realizing contrast and improving response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0168] The preparation of the liquid crystal composition of the present invention is conventionally prepared by a known method, for example, a method of dissolving essential components at high temperature. In addition, additives well known to those skilled in the art, such as optically active compounds or polymerizable compounds described below, can also be added according to the application to prepare the liquid crystal composition of the present invention including a polymerization initiator, adding Liquid crystal composition for guest-host··Guest-Host, GH··type of dye. In general, additives are well known to those skilled in the art, and are described in detail in literature and the like.
[0169] The liquid crystal composition of the present invention may further contain one or more optically active compounds.
[0170] Optically active compounds are added with known chiral dopants. The chiral dopant has the effects of inducing a helical structure of liquid crystals, adju...
example
[0202] [Examples of Liquid Crystalline Compound (1)]
[0203] Hereinafter, the present invention will be described in more detail by examples. However, the present invention is not limited to these examples. In addition, unless otherwise specified, "%" means "wt%". The obtained compound was obtained by using 1 The nuclear magnetic resonance spectrum obtained by H-NMR analysis, and the gas chromatogram obtained by gas chromatography (Gas Chromatography, GC) analysis are used for identification. The measurement method using the above nuclear magnetic resonance spectrum and gas chromatogram is in accordance with the method described below. Furthermore, in each example, C represents crystallization, SA represents smectic A phase, SB represents smectic B phase, SX represents smectic phase whose phase structure has not been analyzed, N represents nematic phase, I represents isotropic phase, The units of the phase transition temperature are all in °C.
[0204] 1 H-NMR analysis:...
example 1
[0211] 4-Ethoxy-2,3,3'-trifluoro-4'-((propylcyclohexyl)methoxy)biphenyl (1-1-8) was synthesized according to the synthetic scheme shown below.
[0212]
[0213] Synthesis of 1-ethoxy-2,3-difluorobenzene (T-2)
[0214] Add sodium hydroxide in the water (400ml) solution of 2,3-difluorophenol (T-1) (195.0g), bromoethane (196.2g) and tetrabutylammonium bromide (TBAB) (24.2g) (75.9 g), heated and stirred at 80° C. for 6 hours under a nitrogen atmosphere. After the reaction was completed, extraction was performed with heptane, and the organic layer was washed with water and saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain a black oily substance. The black oil was purified by distillation, whereby 1-ethoxy-2,3-difluorobenzene (T-1) (230.0 g) was obtained as a colorless oil. The yield was 97%.
[0215] Synthesis of 1-ethoxy-2,3-difluorophenylboronic acid (T-3)
[0216] Compound (T-2) (129.5 g) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com