Composition for whitening and removing freckles and preparation thereof
A technology for whitening and freckle removal and a composition is applied in the field of compositions for whitening and freckle removal, which can solve the problems of large toxic and side effects on the human body, slow freckle lightening effect, and no progress, and achieves a technology with small toxic and side effects, good curative effect, and fast onset of effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
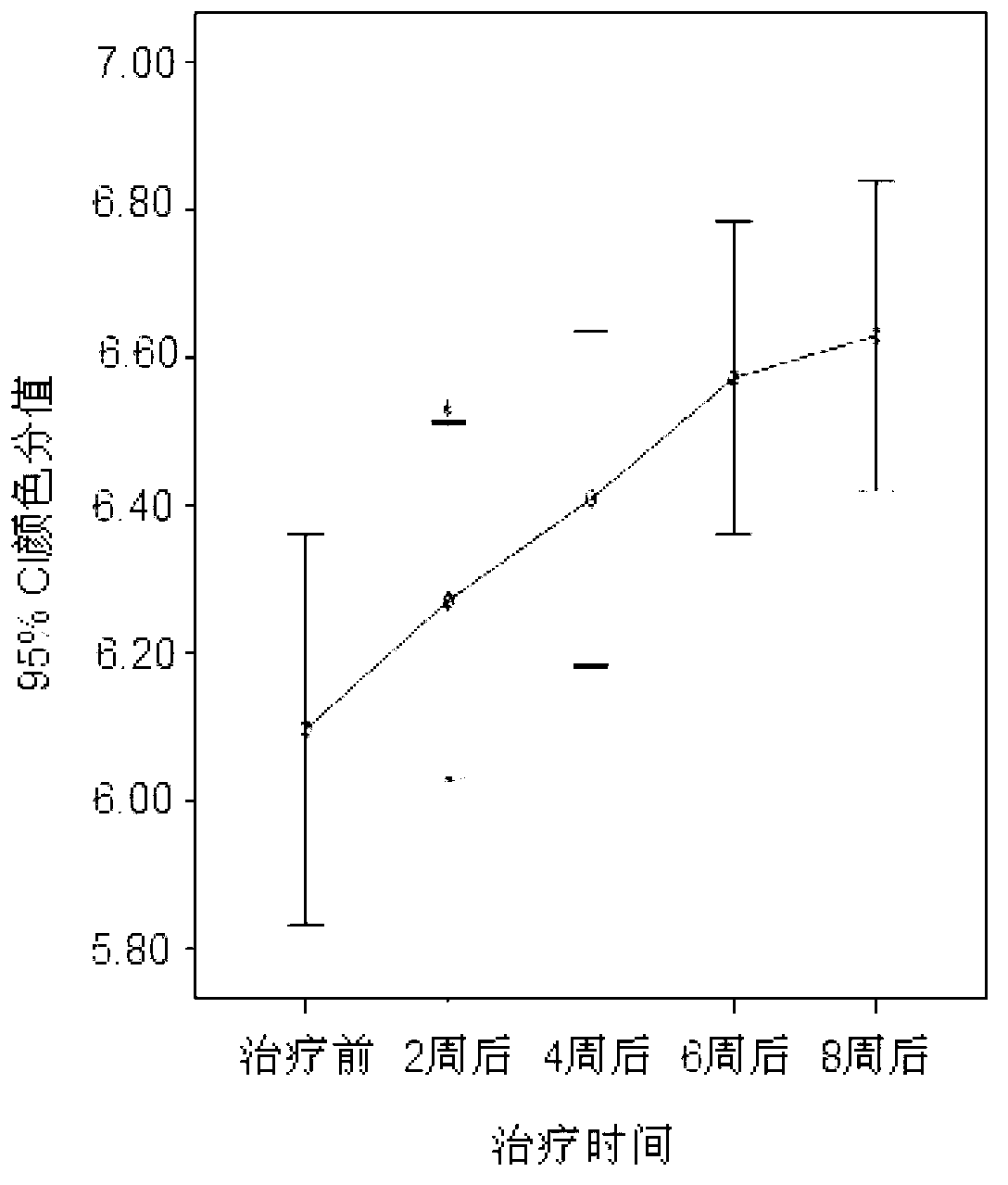
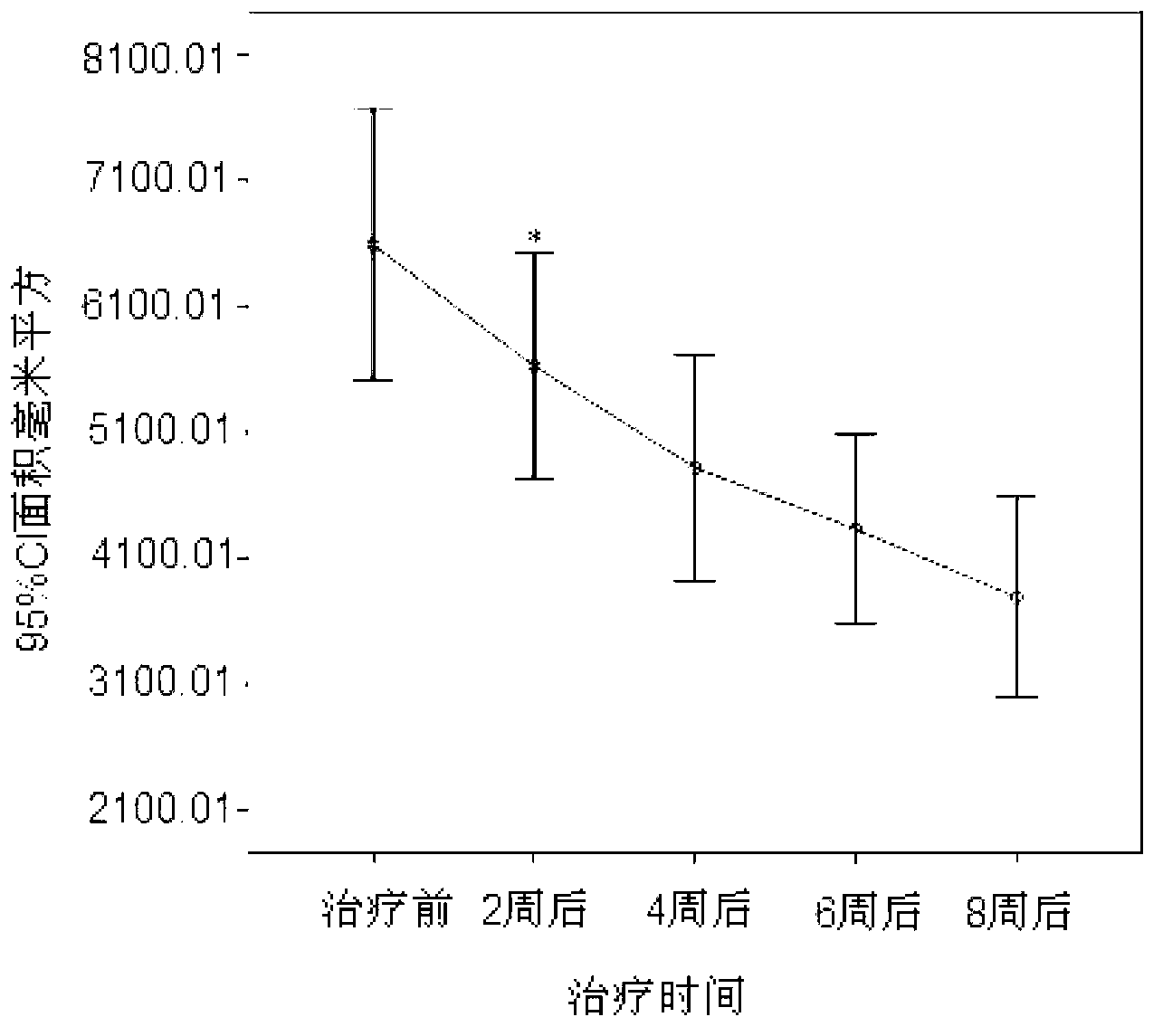
Image
Examples
Embodiment 1
[0044] Active pharmaceutical ingredients include:
[0045] Tranexamic acid 1kg;
[0046] Ascorbic acid tetraisopalmitate 0.5kg;
[0047] Hydrophilic gel matrix includes:
[0048] Sodium polyacrylate 5kg;
[0049] Sodium carboxymethyl cellulose 1kg;
[0050] Gelatin 3kg;
[0051] Glycerin 20kg;
[0052] Aluminum hydroxide 0.05kg;
[0053] Silica 1kg;
[0054] Disodium Edetate 0.08kg;
[0055] Tartaric acid 0.9kg;
[0056] Polysorbate 80 0.3kg;
[0057] 37.17kg of purified water.
[0058] Put 6kg of glycerin into the first container, under stirring, add 1kg of sodium carboxymethylcellulose, and continue stirring until it becomes a translucent colloid, which is component A;
[0059] Take 14kg of glycerin and put it into the second container, add 0.05kg of aluminum hydroxide and 5kg of sodium polyacrylate under stirring, and continue stirring until it becomes a translucent colloid, which is component B;
[0060] Add purified water to the third container, under stirring, add tranexamic acid, ascorbyl t...
Embodiment 2
[0064] Active pharmaceutical ingredients include:
[0065] Tranexamic acid 5kg;
[0066] Ascorbic acid tetraisopalmitate 2kg;
[0067] Hydrophilic gel matrix includes:
[0068] Sodium polyacrylate 6kg;
[0069] Sodium carboxymethyl cellulose 0.8kg;
[0070] Gelatin 3kg;
[0071] Glycerin 25kg;
[0072] Aluminum hydroxide 0.06kg;
[0073] Silica 1kg;
[0074] Disodium Edetate 0.08kg;
[0075] Tartaric acid 0.9kg;
[0076] Polysorbate 80 0.6kg;
[0077] 55.56kg of purified water.
[0078] Put 8kg of glycerin into the first container, under stirring, add 1kg of sodium carboxymethylcellulose, and continue stirring until it becomes a translucent colloid, which is component A;
[0079] Take 17kg of glycerin and put it into the second container, add 0.06kg of aluminum hydroxide and 6kg of sodium polyacrylate under stirring, and continue stirring until it becomes a translucent colloid, which is component B;
[0080] Add purified water to the third container, under stirring, add tranexamic acid, ascorbyl t...
Embodiment 3
[0084] Active pharmaceutical ingredients include:
[0085] Tranexamic acid 2kg;
[0086] Ascorbic acid tetraisopalmitate 0.8kg;
[0087] Hydrophilic gel matrix includes:
[0088] Sodium polyacrylate 5.5kg;
[0089] Sodium carboxymethyl cellulose 1kg;
[0090] Gelatin 3kg;
[0091] Glycerin 25kg;
[0092] Aluminum hydroxide 0.06kg;
[0093] Silica 1kg;
[0094] Disodium Edetate 0.08kg;
[0095] Tartaric acid 0.9kg;
[0096] Polysorbate 80 0.3kg;
[0097] Purified water 60.36kg.
[0098] Put 7 kg of glycerin into the first container, add 1 kg of sodium carboxymethyl cellulose under stirring, and continue stirring until it becomes a translucent colloid, which is component A;
[0099] Take 18 kg of glycerin and put it into the second container, add 0.06 kg of aluminum hydroxide and 5.5 kg of sodium polyacrylate under stirring, and continue stirring until it becomes a translucent colloid, which is component B;
[0100] Add purified water to the third container, under stirring, add tranexamic acid, asco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com